Tunable Ag+ ion release from Ag@C for antibacterial and antifouling performances†
Xiaoliang Yana,
Sha Li*b,
Yunxiang Panc,
Bin Xinga,
Ruifeng Lia,
Ben W.-L. Jangd and
Xuguang Liu*a
aCollege of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China. E-mail: liuxuguang@tyut.edu.cn; yanxiaoliang@tyut.edu.cn; Fax: +86 351 6014138; Tel: +86 351 6014138
bCollege of Textile Engineering, Taiyuan University of Technology, Taiyuan 030024, China
cSchool of Chemistry and Chemical Engineering, Hefei University of Technology, Heifei 230009, China
dDepartment of Chemistry, Texas A&M University-Commerce, Commerce, Texas 75429-3011, USA
First published on 10th April 2015
Abstract
Silver nanoparticles have been regarded as promising candidates in the market as antibacterial agents. However, large production volumes and toxic nature of silver lead to potentially adverse effects on human health and environment, and the rapid consumption of Ag+ ion results in short-term efficacy. In this study, carbon-encapsulated Ag (Ag@C) was designed and used as an antibacterial agent in view of the superior antibacterial property of silver nanoparticles, and a tunable Ag+ ion release rate, which was achieved by varying the thickness of the carbon shell. Structural changes were systematically investigated by a series of experimental and theoretical studies. The results showed the evolution of Ag@C from Ag nanoparticles to triangular Ag nanoplates and finally to carbon-encapsulated hexagonal Ag nanoplates. The antibacterial and antifouling performances of Ag@C towards Escherichia coli/Staphylococcus aureus and Platymonas subcordiformis/Tropidoneis lepidoptera were investigated and the antibacterial mechanism was also discussed. The released Ag+ ion concentrations were controllable and sustainable at 39.4 and 0.667 ppb for Ag@C with carbon shell thicknesses of 31.5 and 247 nm, which attenuated the toxicity of the Ag nanoparticles. Ag@C showed stable antibacterial and antifouling properties and proved to be potentially suitable for biological and environmental applications.
1. Introduction
Marine biofouling is a worldwide problem.1–5 Surfaces immersed in seawater suffer from the settlement of marine organisms, including micro-organisms (bacteria, algae spores) and macro-organisms (barnacles, mussels). This unwanted colonization has a detrimental impact when humans develop and utilize the ocean. Owing to the accumulation of biofouling species on the surface of vessels, fuel consumption increases and maneuvering ability is impaired, leading to the cost of billions of dollars per year in transportation.6 To date, using a marine antifouling coating is the most effective way of avoiding marine organism attachment.Generally, according to the toxicity of coating materials, antifouling strategies can be divided into two main categories: nontoxic coatings and chemically active coatings. The first approach uses nature-inspired structures to inhibit the settlement of organisms without involving chemical reagents. Microtopographical surfaces (marine animal skins) and superhydrophobic surfaces have been employed to defend against biofouling by controlling surface wettability and reducing surface energy. This method physically disrupts the bioadhesion of marine organisms. However, the antifouling properties of these coatings will gradually deteriorate over long time usage under a real marine environment.7,8 Chemically active coatings limit the settlement of marine organisms using chemically active compounds. In the last century, tributyltin (TBT) was the most popular antifouling agent. TBT causes serious damage to the food chain, and the International Maritime Organization (IMO) has forbidden the use of TBT-based agents in marine antifouling coatings. Thus, the development of environmentally friendly antifouling agents is urgently required.
Silver has been extensively used and is well known as a bacteriostatic agent in the treatment of infectious diseases since ancient times. Silver nanoparticles have a broad-spectrum and long-term antibacterial activity and also exhibit low toxicity towards mammalian cells at a small concentration.9,10 Researchers used silver nanoparticles to inhibit marine organisms.11–15 However, there are some disadvantages limiting this antifouling material for further development: (i) large production volumes and toxic nature, which lead to potentially adverse effects on human health and environment; (ii) short-term efficacy, which results from rapid consumption of Ag+ ion; and (iii) poor compatibility with resin, which decreases the uniformity. Based on these facts, the preparation of highly stable Ag nanoparticles with controllable and sustainable Ag+ ion release is a prerequisite for a promising bacteriostatic agent with low toxicity and long-term activity.
Core–shell structural Ag nanocomposites can meet the demands. Many efforts have been made to synthesize core–shell Ag@silica due to the synergetic effects of core Ag and shell materials in antibacterial and antifouling treatments.16–20 In addition, carbon nanoscrolls filled with Ag nanoparticles were prepared by sonication and possessed enhanced and lengthened antifungal activity.21 The combination of graphene oxide (GO) with Ag@Fe2O3 enhanced the stability of Ag nanoparticles and slowed down the Ag+ ion release rate.22 Ag@Fe2O3–GO showed better long-term antibacterial activity than that of plain Ag and Ag@Fe2O3. Therefore, the introduction of auxiliary materials for controlling Ag+ ion release is necessary. Carbon materials have been regarded as promising candidates for Ag encapsulating materials owing to their fine physical/chemical durability and good biological compatibility. First, carbon shells improve the environmental stability of Ag nanoparticles by protecting them from light and moisture. Then, Ag+ ion sustained-release can be controlled by the tunable thickness of the carbon shell, leading to long-term antifouling stability. Finally, the carbon shell can be functionalized and chemically bonded with resin, which can improve the dispersity of Ag nanoparticles.
In this work, a tunable carbon shell encapsulated Ag (Ag@C) nanocomposite was synthesized by changing the hydrothermal reaction time. The growth mechanism of Ag@C was proposed based on experimental and theoretical studies. The general objective of this study is to control the rate of Ag+ ion release by varying the carbon shell thickness and to effectively reduce the impact on the environment. The antibacterial activity (long-term stability) together with the antifouling property was studied. To the best of our knowledge, this type of nanocomposite has not been reported for antibacterial and algal inhibiting performance. The antibacterial mechanism of Ag@C was also discussed.
2. Experimental
2.1. Materials
Glucose, silver nitrite (AgNO3), acetone, and ethanol were commercially available (Tianjin Kemiou Chemical Agent Factory) and of analytical grade without further purification. Algal medium (f/2 seawater medium) was purchased from Shanghai LeadingTec company.2.2. Preparation of Ag@C
A Ag@C core–shell structural nanocomposite was prepared by a hydrothermal method.23 In a typical procedure, a AgNO3 aqueous solution (0.05 moL L−1, 15 mL) was added drop by drop into the glucose solution (0.1 moL L−1, 15 mL) under vigorous stirring. The mixture was ultrasonicated for 10 minutes. Finally, the above-mentioned solution was sealed in a 50 mL Teflon-sealed autoclave. The autoclave was kept at 180 °C for 1–16 h before being cooled in air naturally. After that, the sample was obtained by centrifuging. Three centrifugation/washing/redispersion procedures in acetone, deionized water and alcohol were required before oven drying at 50 °C under vacuum. The obtained products were denoted by the composition and reaction time. Therefore, Ag@C-8 and Ag@C-16 correspond to the Ag and carbon composites obtained from 8 and 16 h reactions, respectively.2.3. Characterizations
Transmission electron microscopy (TEM) observations were carried out using a JEM100CXII operated at 100 kV (a small quantity of sample in ethanol solution was dropped onto the 300 mesh copper TEM grid). Scanning electron microscopy (SEM) images (powder samples) with energy dispersive X-ray spectra (EDS) were recorded with a Hitachi field emission scanning electron microscope (S4800). Diffuse-reflectance UV-Vis (DR UV-Vis) spectroscopy was recorded with a Shimadzu UV-2550 spectrophotometer (three times dilute supernate of samples after hydrothermal process). X-ray diffraction (XRD) analyses were performed with a Rigaku D/MAX-2500 V/PV using Cu-Kα radiation (40 kV and 200 mA) at a scanning speed of 4° min−1 over the 2θ range of 10–70° (powder samples). Raman spectroscopy was used to estimate the presence of carbon and silver species. Spectra were collected with a DXR microscope instrument using a laser with 532 nm wavelength for excitation (powder samples). Fourier transform infrared (FT-IR) spectrometer was recorded using a Tensor 27 spectrometer (Bruker) with a resolution of 4 cm−1 (powder samples with KBr pellet). Thermo-gravimetric analysis (TGA) was carried out using a Netzsch STA 449 F3 system with a heating rate of 10 °C min−1 (from 100 °C to 600 °C) under flowing air (25 mL min−1). A Zetasizer NanoZS90 (Malvern Instruments Ltd., UK) was used to measure and calculate the ζ-potential value of Ag@C solution in water. The Ag+ ion concentration of each sample was quantitatively determined by inductively-coupled plasma mass spectrometry (ICP-MS, Thermo X series 2).2.4. Antibacterial tests
Two representative bacteria Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), which are gram-negative and gram-positive bacteria, respectively were selected as the indicators for antibacterial evaluation. Bacteria were grown in a nutrient agar culture medium (peptone 10 g, beef extract 3 g, NaCl 3 g, distilled water 1000 mL, pH 7.4) and incubated overnight at 37 °C. Then, a culture where bacteria grew in a logarithmic growth phase (18 h) was prepared for an antibacterial test. All disks and materials were sterilized in an autoclave at 120 °C for 30 min before tests. The antibacterial activities of Ag@C samples were performed by disk diffusion assay. The procedure for the disk diffusion assay included (i) placing a 5 mm disk saturated with 20 μL of Ag@C composite aqueous dispersion (500 μg μL−1) onto an agar plate seeded with E. coli or S. aureus and (ii) measuring the diameters of the inhibition zones after incubation for 12 h at 36.8 °C.2.5. Algal inhibiting test
Platymonas subcordiformis (P. subcordiformis) and Tropidoneis lepidoptera (T. lepidoptera) were selected as the indicators for the antifouling evaluation. Algae were placed in the f/2 seawater (filtered by a 0.4 μm microporous membrane) medium and cultured at 20 °C with 3000 lx illumination in the shaking bed. Finally, antifouling agents were added, and the performances were observed under a microscope after 24 h.2.6. Computational methods
Geometry optimization and frequency calculations were performed using density functional theory (DFT) embodied in the Vienna ab simulation package (VASP).24 The surface was modeled by a periodic slab containing four atomic layers with full relaxation of the uppermost two layers. The p (2 × 2) unit cell was used in this study, which means the coverage of adsorbates and pre-adsorbed carbon atoms is 1/4 monolayer (ML). The Monkhorst–Pack meshes of 3 × 3 × 1 K point sampling in the surface Brillouinzone were used.25 The optimized lattice constant of 4.18 Å is used for Ag.3. Results and discussion
3.1. Preparation of Ag@C
TEM observations were conducted to visually illustrate the procedure of Ag@C growth. Fig. 1 presents the morphology evolution of the as-prepared products in the hydrothermal process. Quasi-spherical Ag nanoparticles were obtained at 1 h (Fig. 1a). These Ag nanoparticles had an average size of ∼20 nm. Approximately 50–60 nm triangular Ag nanoplates were observed at 2 h (Fig. 1b). After 4 h (Fig. 1c), thin carbon layer adhered to truncated triangular Ag nanoplates, covered the outer surface and formed a shell of ∼3.3 nm. Well-structured carbon-encapsulated hexagonal Ag nanoplates (Fig. 1d–f) were observed at 8 and 16 h. The spot points (electron diffraction analysis of an individual Ag nanoplate locating flat on the substrate) with a hexagonal arrangement (Fig. 1e) clearly indicated that the particle was a single crystal with its [111] orientation parallel to the electron beam. The size of core Ag nanoplates (Ag@C-8 and Ag@C-16) remained at ∼120 nm, unrelated to the increase of reaction time. However, the thickness of the carbon shell grew from 31.5 to 247 nm, closely correlated with the increase of the reaction time. | ||
| Fig. 1 TEM images of Ag@C at different reaction times: 1 h (a), 2 h (b), 4 h (c), 8 h (d), 16 h (f) and SAED of Ag@C at 8 h (e). | ||
The overall morphology and dispersity of as-prepared Ag@C products (reaction at 8, 16 h) were further characterized by SEM in Fig. 2. It can be clearly seen in Fig. 2a that hexagonal Ag@C-8 nanoplates are well scattered on the substrate. This nanocomposite possessed uniform shape and was highly dispersed. This was attributed to the carbon shell acting as a barrier and preventing Ag from agglomerating. Furthermore, EDS elemental mapping of Ag@C-8 was performed, as shown in Fig. 2c–f, revealing that the Ag@C-8 consisted of Ag, C, and O. Evidently, the blue Ag core was surrounded by the red carbon material and green O. The results were consistent with TEM observations. Fig. 2b presents the SEM images of the product from the 16 h reaction. It was difficult to distinguish the morphology of core Ag from the overall spherical particles, due to the thickness of the carbon shell. However, based on the results of TEM observation, it was reasonable to assume that these spherical nanoparticles (∼600 nm) were assigned to the core–shell structural nanocomposite.
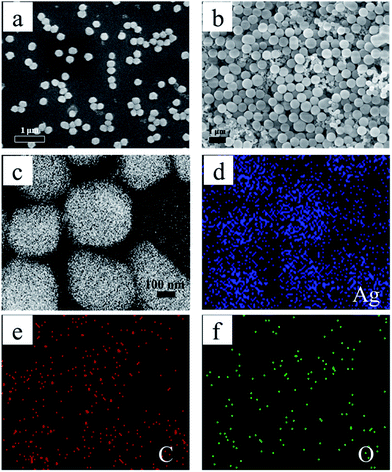 | ||
| Fig. 2 SEM images of Ag@C at different reaction times: 8 h (a), 16 h (b), and FESEM EDS mapping of Ag@C at 8 h (c–f). | ||
UV-Vis spectra of all solutions from 1, 2, 4, and 8 h reactions before centrifugation are shown in Fig. 3. It is well known that the plasmon resonance of nano-structured Ag is sensitive to shape, size, and morphological conversion. According to Mie theory, small spherical Ag nanocrystals exhibit a single surface plasmon band, whereas anisotropic particles show two or three bands, depending on their shape.26 In Fig. 3a, the extinction spectrum of the product from 1 h shows a strong band with a maximum at 418 nm. This was characterized for typical surface plasmon resonance of silver nanoparticles,27 indicating that the reduction of silver ions occurred. For the 2 h reaction product, the absorption spectrum displayed a broad band at 450 nm, together with one weak band at 330 nm. This was ascribed to the presence of triangular Ag nanoplates. It has been widely accepted that the bands at 340 and 470 nm correspond to the plasmon resonance of triangular Ag nanoplates.28,29 This blue shift of the bands from 340 to 330 nm and from 470 to 450 nm was attributed to the following two aspects: (i) the smaller size of triangular Ag nanoplates (∼50–60 nm in this work, while 90 nm in ref. 28); (ii) the evolution of triangular Ag nanoplates into truncated Ag nanoplates.30 In addition, a new band emerged at 295 nm, due to the presence of carbon-containing species (carbon dot).31
As the reaction proceeded to 4 h, a quite different UV-Vis spectrum was observed. The absorption spectrum exhibited three bands at 367, 415, and 492 nm. These three bands can be attributed to the formation of hexagonal Ag nanoplates.28,32 The weak band at 367 nm was assigned to the out-of-plane quadrupole resonance of silver nanoplates. A shoulder band with medium intensity at 415 nm was credited to the out-of-plane dipole resonance of silver nanoplates. A distinctive band (strong intensity) at 492 nm was assigned to the in-plane dipole resonance of silver nanoplates. Thus, it was confirmed that the triangular Ag nanoplates became truncated to evolve into hexagonal Ag nanoplates. Furthermore, in comparison with the absorption spectrum shown in Fig. 3b for a 2 h reaction product, the band intensity of the carbon species was enhanced for the product obtained at 4 h, based on the calculation of the intensity ratio of the bands between the carbon and Ag product. This probably indicated the formation of a relatively large amount of carbon species on the surface of hexagonal Ag nanoplates. After the reaction had proceeded for 8 h (Fig. 3d), the bands eventually disappeared from the visible regime. Only one clear band at 311 nm remained. It can be deduced that one group of nano-structural Ag was formed and was surrounded by a large number of carbon shells. These findings allow us to draw some conclusions about the formation and evolution of Ag@C: (i) the glucose solution can reduce the silver nitrate and the shape of Ag changed from nanoparticles to triangular nanoplates and then to hexagonal nanoplates; (ii) the remaining glucose (after the process of reduction of the silver precursor) adhered and carbonized on the surface of Ag, leading to the formation of carbon-encapsulated Ag.
XRD patterns of Ag@C obtained from 4, 8, and 16 h reactions are shown in Fig. 4a. It can be observed that the crystal phase of the samples was composed of carbon and silver. Diffraction peaks observed at 2θ = 38.2°, 44.4°, and 64.6° were indexed to Ag (111), Ag (200), and Ag (220), which was in agreement with the reported values from the Joint Committee on Powder Diffraction Standards card (JCPDS, no. 04-0783). The residual board peak at a 2θ degree = 25.2° corresponded to the (002) crystal plane of carbon. This suggested the presence of graphite carbon with low crystallinity.
Fig. 4b shows the Raman spectra of Ag@C obtained from 4, 8, and 16 h reactions. The Raman spectrum of as-obtained sample from 4 h presented typical bands of Ag (four bands at 556, 842, 987, and 1071 cm−1).33 One weak band at 1580 cm−1 was assigned to the G peak of carbon. The band intensity of Ag was greatly larger than that of carbon. With increasing reaction time, the band intensity of Ag significantly decreased and even disappeared, whereas the band intensity of carbon significantly increased. An additional band at 1380 cm−1 (disorder-induced D peak) emerged as the reaction time increased, indicating the low crystallinity of the carbon.
3.2. Characterizations of Ag@C-8 and Ag@C-16
The surface properties (functional groups) and thermal stability (Ag loading content) of Ag@C were studied using FT-IR and TGA. Fig. 5a illustrates the FT-IR spectra of Ag@C-8 and Ag@C-16. The band centered at ∼3500 cm−1 was ascribed to the single bond –OH stretching vibrations. The signature bands at 1700 and 1615 cm−1 corresponded to the C double bond, caused by the aromatization of glucose during the hydrothermal treatment. The absorption bands from 1000 to 1300 cm−1 were attributed to the C single bond –OH stretching and –OH bending vibrations, suggesting the existence of large numbers of residual hydroxyl groups. Partially dehydrated residues, in which reductive –OH and –CHO were covalently bonded to the carbon frameworks, improved the hydrophilicity and stability of the nanospheres in aqueous systems, which would greatly expand their potential application range in biochemistry, diagnostics, and drug delivery.Fig. 5b shows the TG-DTA profiles of Ag@C-8 and Ag@C-16. The profiles of both samples exhibited one evident stage of weight loss as a result of the combustion of carbon. The weight loss for Ag@C-16 was greater in comparison with that of Ag@C-8. Furthermore, the DTA peak of Ag@C-16 (449 °C) shifted to a higher temperature compared with that of Ag@C-8 (389 °C). This was mainly ascribed to the large amount of carbon gained with increasing reaction time, leading to a higher combustion temperature for carbon. The remaining weight percentages (Ag loading content) of Ag@C-8 and Ag@C-16 were 30.2% and 13.3%, respectively. This suggested that Ag@C-8 had a higher ratio of silver to carbon.
3.3. Mechanism of formation of Ag@C
According to the results of TEM, SEM, UV-Vis, XRD and Raman characterizations, the growth model for Ag@C is proposed in Fig. 6a. At the very beginning, the reduction of silver ions by glucose occurred, leading to the thermodynamically favorable formation of Ag nanoparticles. These nanoparticles had structural defects, as is shown in Fig. 1a. While the intermediate state (formation of Ag nanoparticles) was unstable, these nanoparticles would grow large or evolve into an anisotropic morphology within the scope of kinetic control. As the reaction proceeded, these Ag nanoparticles grew larger and evolved into Ag triangular nanoplates. Xia and co-workers reported a kinetically controlled synthesis of Ag triangular nanoplates using the mild reducing reagent, PVP.34 With a slow reduction rate, the nucleation and growth of Ag was in a kinetic control region, whereas a fast reduction rate led to the growth of other structures instead of nanoplates. Moreover, they pointed out the significant influence of structural defects in the formation of nanoplates. The presence of defects in the initial seed played an important role in the anisotropic growth of triangular nanoplates.35 Thus, in this work, the morphology transformation of Ag nanoparticles into Ag triangular nanoplates was caused by the structural defects of Ag nanoparticles and the kinetically controlled reduction of Ag species by glucose. | ||
| Fig. 6 (a) The growth mechanism of Ag@C. (b) The interaction model of atomic carbon with Ag crystal planes: Ag (111) (left) and Ag (100) (right). | ||
These Ag triangular nanoplates became truncated and evolved into hexagonal Ag nanoplates. In order to explain the morphology change of Ag nanoplates, DFT method was applied to investigate the binding energies of atomic C on Ag (111) and Ag (100) facets, separately. Ag surfaces were modeled with a periodic array of four-layer-thick slabs. The interaction models of atomic C with Ag (111) and Ag (100) are shown in Fig. 6b. The adsorption energy (Eads) is calculated by the formula Eads = EA/M − EA − EM. EA, EM, and EA/M are the calculated energies of adsorbate, substrate, and adsorption system, respectively.
The calculated results showed that atomic C prefers to adsorb at a hollow site, even though its original adsorb sites were top or bridge. The calculated adsorption energies of atomic C on Ag (100) and Ag (111) were −4.41 eV and −3.33 eV, respectively. This indicated that the carbon from the glucose decomposition could prefer to interact with the Ag (100) surface, and could inhibit the growth of the surface. As a result, the growth of the sample along the (100) direction was stopped by the deposited carbon and forms a step structure. As for the Ag (111) surface, due to the relatively weak interaction between the surface and the carbon atoms, Ag atoms can easily enter into the interface between the surface and the carbon atoms. This may help the growth of the Ag (111) surface. Owing to the excess amount of glucose, the as-obtained hexagonal Ag nanoplates were encapsulated by carbon shells through the carbonization of glucose.
3.4. Antibacterial property of Ag@C
The antibacterial activities of Ag@C-8 and Ag@C-16 were evaluated by determining the presence of inhibition zones. The antibacterial agent was used in water solution, and the stability of the solution was analyzed by ζ-potential analysis. The ζ-potential value of Ag@C in water (100 ppm) was measured. The value was large and negative (−45.0 mV), indicating the stability of the solution. Antibacterial effects in the form of inhibition zones, assessed by the disk diffusion of Ag@C-8 and Ag@C-16, are shown in Fig. 7a. As expected, the normal growth of E. coli and S. aureus was seen in the agar plate when no composites were introduced. The blank experiment with only filter paper demonstrated almost no antibacterial activity without the addition of Ag@C. Both Ag@C-8 and Ag@C-16 exhibited relatively strong antibacterial activity, as depicted from the inhibition zone. The diameters of the inhibition zones from Ag@C-8 and Ag@C-16 on S. aureus/E. coli were 13.2/11.8 mm and 10.4/9.3 mm, respectively. It can be seen that Ag@C-8 exhibited a better antibacterial performance than Ag@C-16. The antibacterial activity for the repeated use of Ag@C was investigated (Fig. 7b). The diameters of the inhibition zones from Ag@C-8 and Ag@C-16 were almost unchanged, indicating stable antibacterial activity. | ||
| Fig. 7 (a) The inhibition zone test for S. aureus and E. coli of blank, Ag@C-8 and Ag@C-16. (b) Antibacterial activity for repeated use of Ag@C. | ||
The antibacterial mechanism of Ag nanoparticles has not been completely understood. However, many studies demonstrated that the released Ag+ ion from Ag nanoparticles plays a crucial role in antibacterial performance.14–22,36 Hurt and co-workers have reported that Ag0 nanoparticles would not last long in realistic environment containing dissolved oxygen through thermodynamic analysis and kinetic measurements. H+ and dissolved O2 oxidized Ag nanoparticles to release Ag+ ion.36 When Ag@C acted as the antifouling agent in seawater, Ag+ ion was released from the Ag core and penetrated through the carbon shell to make contact with fouling organisms. Ag+ ion concentrations of Ag@C-8 and Ag@C-16 in water (the above reference mentioned sea salts had only a minor effect on dissolved silver release) were determined by ICP-MS. Fig. 8a shows the Ag+ ion release profiles of Ag@C-8 and Ag@C-16. The released Ag+ ion concentrations were 39.4 and 0.667 ppb for Ag@C-8 and Ag@C-16, respectively, in the first run and were subsequently maintained at these levels during the five-cycle test. These concentrations were below the standards of the US Environmental Protection Agency (US EPA) and the World Health Organization (WHO) for drinking water (54 ppb).37 In addition, the carbon shell exhibits relatively low toxicity. Similarly, using the hydrothermal approach, carbon spheres were obtained.38 The resultant carbon nanospheres showed low cytotoxicity and when the concentration increased to 100 μg mL−1, the cell viability remained above 65%. Furthermore, studies on the effects of carbon-coated ZnO nanorods and pure ZnO nanorods on cultured mouse fibroblast cells revealed that the coating of biocompatible carbon significantly reduced the cytotoxicity of ZnO nanorods.39
 | ||
Fig. 8 (a) The Ag+ ion release rate of Ag@C-8 ( ) and Ag@C-16 ( ) and Ag@C-16 ( ). (b) Schematic of the antibacterial process of Ag@C. ). (b) Schematic of the antibacterial process of Ag@C. | ||
Fig. 8b was used to illustrate the antibacterial process of Ag@C. Ag@C had abundant pores and channels, which supplied the route for Ag+ ion penetration. Hydroxyl inherited from glucose has been grafted onto the channels after hydrothermal treatment, which was beneficial to the adsorbance of Ag+ ion, making the pores of Ag@C function as Ag+ ion reservoirs. Then the dissolved Ag+ ion bounded to thiol groups in protein and interfered with DNA replications, inducing the inactivation of the bacterial proteins. With the consumption of Ag+ ion by bacteria, fresh Ag+ ion was replenished. The thickness of the carbon shell was a dominant factor for tuning Ag+ ion release. The thickness of the shell determined the pathways of Ag+ ion release. The thicker shell of Ag@C-16 (247 nm, Fig. 1f) compared to that of Ag@C-8 (31.5 nm, Fig. 1d) contributes to the longer pathways, leading to a significant reduction of the diffusion rate of Ag+ ion. Moreover, hydroxyl concentration was in direct proportion to the thickness of the shell. Ag@C-16 had a larger carbon shell density, which produced plentiful amounts of hydroxyl and reserved a large number of Ag+ ion. If 100 ppm of Ag@C solution was employed, the complete exhausting of Ag+ ion over Ag@C-8 would last for about 2 years and for almost 55 years for Ag@C-16. Thus, Ag@C (especially Ag@C-16) possessed a continuous and controllable release feature, which was the fundamental character of stable, long-term efficiency as an antibacterial agent and reduced adverse effects in application.
3.5. Algal inhibiting property of Ag@C
Platymonas subcordiformis (P. subcordiformis) and Tropidoneis lepidoptera (T. lepidoptera) in seawater were selected as representative microalgae, in order to fully evaluate the practical use of Ag@C as an antifouling material. Fig. 9 shows the microscopic images for algal inhibiting properties. In fresh seawater, P. subcordiformis was a type of active (Fig. 9) and motile (Movie S1†) green algae, whereas T. lepidoptera can firmly adhere to and settle on the substrate surface. Much less P. subcordiformis was observed on 100 ppm Ag@C-8 and Ag@C-16 (Movie S2†). In addition, with the Ag content increased to 300 ppm, a large proportion of T. lepidoptera was inhibited and killed, as can be seen from the empty area in Fig. 9, by Ag@C-8 and Ag@C-16. The results were in accordance with the stable performance and long-term efficiency of antibacterial behavior. | ||
| Fig. 9 The fluorescence microscope photos for P. subcordiformis (100 ppm antifouling agent) and T. lepidoptera (300 ppm antifouling agent) of blank, Ag@C-8 and Ag@C-16 test. | ||
4. Conclusions
In summary, a method for controllable release of Ag+ ion was proposed by the synthesis of carbon-encapsulated Ag for antibacterial and antifouling applications. Experimental and theoretical studies demonstrated the growth procedure of Ag nanoparticles into Ag triangular nanoplates and finally into carbon-encapsulated hexagonal Ag nanoplates. The thickness of the carbon shells played a vital role in the pathways of Ag+ ion release. Ag@C showed good antibacterial activity and antifouling performance towards E. coli/S. aureus and P. subcordiformis/T. lepidoptera, respectively. In addition, Ag@C exhibited low released Ag+ ion concentrations below the standards of US EPA and WHO for drinking water. The results showed that core–shell structural Ag@C gave new opportunities for antibacterial and antifouling materials with high efficiency, low toxicity, and long-term stability. Furthermore, this method for the tunable release of Ag+ ion can be used for the controllable release of other metal ions.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (21406153) and the Shanxi Province Science Foundation for Youths (2014021014-2). The authors are also grateful for help with investigation of antifouling performance by the China Shipbuilding Industry Corporation (no. 725 Institute in Xiamen City).References
- J. A. Callow and M. E. Callow, Nat. Commun., 2011, 2, 244–254 CrossRef PubMed.
- M. Lejars, A. Margaillan and C. Bressy, Chem. Rev., 2012, 112, 4347–4390 CrossRef CAS PubMed.
- C. F. Ma, L. G. Xu, W. T. Xua and G. Z. Zhang, J. Mater. Chem. B, 2013, 1, 3099–3106 RSC.
- A. K. Singh, P. Singh, S. Mishra and V. K. Shahi, J. Mater. Chem., 2012, 22, 1834–1844 RSC.
- P. M. Imbesi, N. V. Gohad, M. J. Eller, B. Orihuela, D. Rittschof, E. A. Schweikert, A. S. Mount and K. L. Wooley, ACS Nano, 2012, 6, 1503–1512 CrossRef CAS PubMed.
- M. P. Schultz, J. A. Bendick, E. R. Holm and W. M. Hertel, Biofouling, 2011, 27, 87–98 CrossRef CAS PubMed.
- A. J. Scardino and R. de Nys, Biofouling, 2010, 27, 73–86 CrossRef PubMed.
- L. D. Chambers, K. R. Stokes, F. C. Walsh and R. J. K. Wood, Surf. Coat. Technol., 2006, 201, 3642–3652 CrossRef CAS PubMed.
- J. Kusnetsov, E. Iivanainen, N. Elomaa, O. Zacheus and P. J. Martikainen, Water Res., 2001, 35, 4127–4225 CrossRef.
- Z. Z. Li, L. J. Fan, T. Zhang and K. Li, J. Hazard. Mater., 2011, 187, 466–472 CrossRef CAS PubMed.
- T. S. Sileika, H.-D. Kim, P. Maniak and P. B. Messersmith, ACS Appl. Mater. Interfaces, 2011, 3, 4602–4610 CAS.
- P. Gunawan, C. Guan, X. H. Song, Q. Y. Zhang, S. S. J. Leong, C. Tang, Y. Chen, M. B. Chan-Park, M. W. Chang, K. Wang and R. Xu, ACS Nano, 2011, 5, 10033–10040 CrossRef CAS PubMed.
- T. Q. Tuan, N. V. Son, H. T. K. Dung, N. H. Luong, B. T. Thuy, N. T. V. Anh, N. D. Hoa and N. H. Hai, J. Hazard. Mater., 2011, 193, 1321–1329 CrossRef PubMed.
- M. S.-L. Yee, P. S. Khiew, Y. F. Tan, Y.-Y. Kok, K. W. Cheong, W. S. Chiu and C.-O. Leong, Colloids Surf., A, 2014, 457, 382–391 CrossRef CAS PubMed.
- D. Inbakandan, C. Kumar, L. Stanley Abraham, R. Kirubagaran, R. Venkatesan and S. Ajmal Khan, Colloids Surf., B, 2013, 111, 636–643 CrossRef CAS PubMed.
- P. Saint-Cricq, J. Z. Wang, A. Sugawara-Narutaki, A. Shimojima and T. Okubo, J. Mater. Chem. B, 2013, 1, 2451–2454 RSC.
- C. Durucan and B. Akkopru, J. Biomed. Mater. Res., Part B, 2010, 93, 448–458 CrossRef PubMed.
- G. Fuertes, O. L. Sánchez-Muñoz, E. Pedrueza, K. Abderrafi, J. Salgado and E. Jiménez, Langmuir, 2011, 27, 2826–2833 CrossRef CAS PubMed.
- S. K. Das, M. M. R. Khan, T. Parandhaman, F. Laffir, A. K. Guha, G. Sekaran and A. B. Manda, Nanoscale, 2013, 5, 5549–5560 RSC.
- X. S. Zhang, J. X. Wang, K. Xu, Y. Le and J. F. Chen, J. Nanosci. Nanotechnol., 2011, 11, 3481–3487 CrossRef CAS PubMed.
- C. Li, X. S. Wang, F. Chen, C. L. Zhang, X. Zhi, K. Wang and D. X. Cui, Biomaterials, 2013, 34, 3882–3890 CrossRef CAS PubMed.
- N. Gao, Y. J. Chen and J. Jiang, ACS Appl. Mater. Interfaces, 2013, 5, 11307–11314 CAS.
- X. M. Sun and Y. D. Li, Langmuir, 2005, 21, 6019–6024 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- G. Mie, Ann. Phys., 1908, 25, 377–445 CrossRef CAS PubMed.
- J. J. Mock, M. Barbic, D. R. Smith, D. A. Schultz and S. Schultz, J. Chem. Phys., 2002, 116, 6755–6759 CrossRef CAS PubMed.
- R. C. Jin, Y. W. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Science, 2001, 294, 1901–1903 CrossRef CAS PubMed.
- J. Yang, Q. B. Zhang, J. Y. Lee and H.-P. Too, J. Colloid Interface Sci., 2007, 308, 157–161 CrossRef CAS PubMed.
- S. H. Chen and D. L. Carroll, Nano Lett., 2002, 2, 1003–1007 CrossRef CAS.
- Q. L. Wang, H. Z. Zheng, Y. J. Long, L. Y. Zhang, M. Gao and W. J. Bai, Carbon, 2011, 49, 3134–3140 CrossRef CAS PubMed.
- J. An, B. Tang, X. H. Ning, J. Zhou, S. P. Xu, B. Zhao, W. Q. Xu, C. Corredor and J. R. Lombardi, J. Phys. Chem. C, 2007, 111, 18055–18059 CAS.
- G. I. N. Waterhouse, G. A. Bowmaker and J. B. Metson, Phys. Chem. Chem. Phys., 2001, 3, 3838–3845 RSC.
- I. Washio, Y. Xiong, Y. Yin and Y. Xia, Adv. Mater., 2006, 18, 1745–1749 CrossRef CAS PubMed.
- T. C. R. Rocha and D. Zanchet, J. Phys. Chem. C, 2007, 111, 6989–6993 CAS.
- J. Y. Liu and A. H. Hurt, Environ. Sci. Technol., 2010, 44, 2169–2175 CrossRef CAS PubMed.
- B. Zhang, J. Sun, C. Bi, G. Yin, L. Pu, Y. Shi and L. Sheng, New J. Chem., 2011, 35, 849–853 RSC.
- Y. Fang, D. Gu, Y. Zou, Z. X. Wu, F. Y. Li, R. C. Che, Y. H. Deng, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7987–7991 CrossRef CAS PubMed.
- Y. Guo, H. S. Wang, C. L. He, L. J. Qiu and X. B. Cao, Langmuir, 2009, 25, 4678–4684 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02904j |
| This journal is © The Royal Society of Chemistry 2015 |



