A comparative study on the electron transfer reaction (ETR) of surfactant cobalt(iii) complexes of aliphatic/aromatic ligands in micro heterogeneous media: a thermodynamic approach†
Abstract
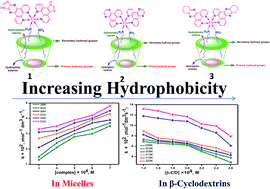
The kinetics of the electron transfer reaction (ETR) between the surfactant cobalt(III) complex ions, cis-[Co(ip)2(C12H25NH2)2](ClO4)3, cis-[Co(dpq)2(C12H25NH2)2](ClO4)3 and cis-[Co(dpqc)2(C12H25NH2)2](ClO4)3 (ip = imidazo[4,5-f][1,10]phenanthroline, dpq = dipyrido[3,2-d:2′-3′-f]quinoxaline, dpqc = dipyrido[3,2-a:2′,4′-c](6,7,8,9-tetrahydro)phenazine, C12H25NH2 = dodecylamine) and Fe2+ ions in micelles as well as β-cyclodextrin (β-CD) were studied at different temperatures by a spectrophotometric method under pseudo first order conditions with an excess of reductant. The results from surfactant complexes containing aromatic ligands, which have a higher ETR than that of aliphatic ligands due to the results obtained, have been explained based on the hydrophobic effect. Experimentally the reactions were found to be second order and the electron transfer is postulated as outer sphere. The rate constant increases with increase in the concentration of micelles but the inclusion of the long aliphatic chain of the surfactant cobalt(III) complexes into β-cyclodextrin decreases the rate of the reaction. Thermodynamic parameters were also evaluated.

 Please wait while we load your content...
Please wait while we load your content...