The salt effect on the yields of trioxane in reaction solution and in distillate
Abstract
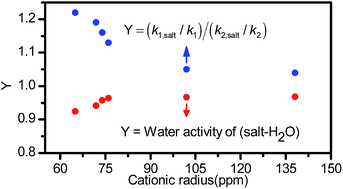
Batch reaction experiments were performed to investigate the salt effect on the yield of trioxane in the reaction solution. The salts considered include NaHSO4, Na2SO4, NaH2PO4, Na2HPO4, KCl, NaCl, LiCl, ZnCl2, MgCl2, and FeCl3. The effects of the anionic structure and the cation charge density on the yield of trioxane in the reaction solution were elucidated and the mechanisms that govern such effects were established. It is shown that the first four salts exerted a negative effect on the yield of trioxane in the reaction solution and such an effect increased progressively from left to right. This trend is due to the formation of NaHSO4, H3PO4, or (H3PO4 and NaH2PO4), which decreased the concentration of H+ in the solution. The latter six salts showed a positive effect on the yield of trioxane in the reaction solution. The salt effect paralleled the ability of the salt to decrease the water activity of the reaction solution and followed the order KCl < NaCl < LiCl < ZnCl2 < MgCl2 < FeCl3. Continuous production experiments were performed to investigate the salt effect on the concentration of trioxane in the distillate. The salts considered were KCl, NaCl, LiCl, ZnCl2, MgCl2, and FeCl3, and the salt effect increased progressively from left to right. Such an effect was shown to be determined by the ability of the salt to increase the yield of trioxane in the reaction solution and to increase the relative volatilities of trioxane and water and of trioxane and oligomers.

 Please wait while we load your content...
Please wait while we load your content...