Synthesis, characterization, thermal behavior, and biological activity of ozonides from vegetable oils†
Nathália Rodrigues de Almeida Kogawa*a,
Eduardo José de Arrudab,
Ana Camila Michelettia,
Maria de Fatima Cepa Matosd,
Lincoln Carlos Silva de Oliveiraa,
Dênis Pires de Limaa,
Nadia Cristina Pereira Carvalhoc,
Paola Dias de Oliveiraa,
Marillin de Castro Cunhad,
Mariah Ojedad and
Adilson Beatriz*a
aInstituto de Química – INQUI, Universidade Federal do Mato Grosso do Sul, Av. Senador Felinto Müller, no. 1555, Cidade Universitária, 79074-460, Campo Grande, MS, Brazil. E-mail: nathrodrigues@live.com; adilson.beatriz@ufms.br; Fax: +55 67 3345 3552; Tel: +55 67 33453676
bFaculdade de Ciência Exatas e Tecnologia (FACET), Universidade Federal da Grande Dourados (UFGD), Itahum, km 12 – Cidade Universitária, 79804-970, Dourados, MS, Brazil
cNúcleo do Hospital Universitário da Universidade Federal do Mato Grosso do Sul – NHU/UFMS, Av. Senador Felinto Müller, S/N, Cidade Universitária, 79.002-970, Campo Grande, MS, Brazil
dLaboratório de Biologia Molecular e Culturas Celulares, Centro de Ciências Biológicas e da Saúde, Universidade Federal de Mato Grosso do Sul, Cidade Universitária S/N. CEP, 79070-900 Campo Grande, MS, Brasil
First published on 27th July 2015
Abstract
In this work, we have synthesized ozonides from sunflower, flaxseed and baru oils. In addition, ozonolysis reaction of sunflower oil in the presence of water was performed, and the product obtained had high viscosity and a gel-like appearance. The ozonated products were investigated for their antimicrobial activity and cytotoxicity. The oleogel, with an MIC ≤ 3 mg mL−1, exhibited excellent antimicrobial activity against standard and clinical strains. All products showed no cytotoxicity when tested against NIH/3T3 murine fibroblast cells. Effects of ozonation time on the oils were analyzed by IR, 1H and 13C NMR spectroscopy. DSC analysis shows that the ozonides of vegetable oils decompose with a peak at about 150 °C and with a broad exotherm. The decomposition enthalpy is proportional to the degree of ozonation reached.
1. Introduction
Ozonated vegetable oils have been the focus of great pharmaceutical interest for the treatment of dermatological disorders, such as infections of skin ulcers and chronic wounds.1,2 Ozonated oils and ozone have been reported as effective in the treatment of refractory wounds, where conventional treatments and available medications prove ineffective.3 In addition, with well-defined concentrations of peroxide, they are used topically to treat anaerobic infections, herpes, ulcers, burns, infections caused by fungi and decubitus ulcers.1,4Ozone reacts with the double bonds of triglycerides present in vegetable oils, forming ozonides and peroxidic species responsible for antimicrobial activity, stimulating regeneration and tissue repair.5 However, a recent study suggested that not only the ozonation grade is important to improve the healing process of the wound but also the typical composition of the oil.2
Ozonated oils from sunflower, olive, sesame, soybean and linseed oils are the most extensively studied with respect to their biological1–12 and physicochemical5,13–19 properties. Other products, such as ozonated grape seed,19 theobroma,20 coconut,21 jojoba,22 rapeseed and corn23 oils have also been investigated.
The ozone molecule acts as a dipole with electrophilic and nucleophilic properties. It can react selectively with organic compounds by direct oxidation of aliphatic unsaturated compounds and aromatic rings.24 Ozonolysis reaction is one of the cleanest and most reproducible reactions and has been widely used in academic research and industrial settings. In 1975, Criegee devoted attention to the ozonolysis reaction, proposing a mechanism for the formation of oxygenated products.25,26
These products have been used in many countries for their medicinal properties. Therefore, knowledge of the dynamics of by-product formation is essential to measure the degree of ozonation required to obtain the desired therapeutic effects.19 Spectroscopic techniques of NMR (1H and 13C) and infrared13–16,18 have been extensively used for characterization of these ozonated oils, associated with simple analytical techniques such as peroxide value,14–18 iodine value,16,17 viscosity determination,13,15–17 and chromatography.15,18
Thermal analysis can be a suitable technique for studying the physical and chemical properties of ozonated oils as raw material or as ingredients in pharmaceutical and cosmetic formulations. However, to the best of our knowledge only a few studies have reported thermochemical approaches of ozonized vegetable oils. Soriano et al.27 measured the decomposition enthalpy of ozonized sunflower oil methyl ester and, Cataldo,28 described ethyl oleate as a simple model of fats, which was submitted to ozonolysis monitored by thermal decomposition enthalpy as function of the ozonation time using DSC (differential scanning calorimetry).
The aim of the present investigation was to perform detailed chemical characterization of ozonated sunflower, flaxseed and baru oils,29–31 as well as of a novel product (oleogel) obtained from ozonolysis reaction of sunflower oil in the presence of water. This work comprises a description of the thermal behavior by DSC of these ozonated products and their characterization by NMR (1H/13C) and IR spectroscopy, and determination of peroxide- and iodine values. The antimicrobial activity against multiresistant strains and the cytotoxicity against neoplastic and non-neoplastic cells were also evaluated.
2. Experimental procedure
2.1. Material and equipment
Flaxseed and baru oils extracted by cold pressing (Veris Óleos Vegetais), as well as refined sunflower oil, were used for the ozonolysis reactions. Ozone gas was generated by a Philozon bench generator at a constant flow rate of 1 L min−1, fed with medicinal oxygen.NMR spectra were recorded in CDCl3 solution on a Bruker DPX300 spectrometer operating at 300/75 MHz for 1H/13C, respectively. The spectra were referenced to TMS using residual solvent signals as secondary standards. Infrared spectra were acquired on film using a Bomen MB100 FT-IR Spectrometer.
DSC analyses were performed on a DSC-Q20 with a RCS-90 device (TA Instruments) at a heating rate of 10 °C min−1 and temperatures from −80 to 500 °C, in a N2 atmosphere at a flow rate of 50 mL min−1 as a purge gas, using an aluminum sealed crucible with perforated cover as support.
2.2. General ozonation procedure
The O2/O3 mixture was bubbled in glass reactors containing 200 mL samples of vegetable oil (sunflower, flaxseed or baru) and the reactions were run at room temperature for 6, 12, 24, and 36 h. The reaction with sunflower oil employed a mixture of oil and water (9% v/v) over a period of 24 h. The products were bubbled with N2 for 5 min and stored at 2–8 °C.2.3. Physicochemical analysis
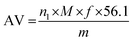 | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and 3 mL of a saturated solution of potassium iodide were added to an Erlenmeyer flask containing 2 g of sample. The mixture was stirred, left under light for 30 h,35 and 25 mL of recently boiled distilled water at room temperature was subsequently added. Titration was carried out with a standardized 0.1 M sodium thiosulfate solution until the solution turned yellowish, at which point 1 mL of 1% starch indicator solution was added and titration continued until disappearance of the deep blue color.36 Iodine analyses were performed in triplicate and the results expressed as standard deviations (±SD). The values were calculated using eqn (3):
2) and 3 mL of a saturated solution of potassium iodide were added to an Erlenmeyer flask containing 2 g of sample. The mixture was stirred, left under light for 30 h,35 and 25 mL of recently boiled distilled water at room temperature was subsequently added. Titration was carried out with a standardized 0.1 M sodium thiosulfate solution until the solution turned yellowish, at which point 1 mL of 1% starch indicator solution was added and titration continued until disappearance of the deep blue color.36 Iodine analyses were performed in triplicate and the results expressed as standard deviations (±SD). The values were calculated using eqn (3):
 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and 3 mL of potassium iodide saturated solution were added to an Erlenmeyer flask containing 2 g of oil. After 180 min of heating at 60 °C, the peroxide reacted with potassium iodide. The samples were cooled to room temperature and 25 mL of recently boiled distilled water was then added. Titration and calculation of peroxide value were performed as previously described.
2), and 3 mL of potassium iodide saturated solution were added to an Erlenmeyer flask containing 2 g of oil. After 180 min of heating at 60 °C, the peroxide reacted with potassium iodide. The samples were cooled to room temperature and 25 mL of recently boiled distilled water was then added. Titration and calculation of peroxide value were performed as previously described.2.4. Antimicrobial activity
Antimicrobial activity assays of the ozonated oils were carried out at the Laboratory of Bacteriology of the Center for Clinical Analysis of the UFMS Teaching Hospital, in Campo Grande, Brazil, using six bacterial strains: Pseudomonas aeruginosa ATCC 27853, Enterococcus faecalis ATCC 29212, E. faecalis clinical strain, Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, and S. aureus clinical strain. The clinical strains were isolated from patients of the UFMS Teaching Hospital.All strains were grown on Mueller–Hinton agar. The inoculants were prepared in sterile 0.85% saline solution. Final inoculum concentrations were read on a DensiChek Plus device (bioMérieux) with a value of 0.5 on the McFarland scale, equivalent to 104 cfu mol−1. Minimum inhibitory concentration (MIC) values were determined using the agar dilution method, according to NCCLS.38 The ozonated oils were solubilized in Mueller–Hinton agar with 2% Tween-80 to increase oil solubility in agar. Test plates were prepared with different ozonated oil concentrations (10, 7, 5, and 3 mg mL−1) and control plates with agar and Tween 80. Aliquots of 5 μL of each inoculum were applied and the plates incubated at 30 °C and read after 24 h.
2.5. Cytotoxic tests
Cytotoxic activity was evaluated in six human neoplastic cell lines—namely, 786-0 (ATCC-CRL-1932, renal adenocarcinoma), HT-29 (ATCC-HTB-38 colon adenocarcinoma), MFC-7 (ATCC-HTB-22, breast adenocarcinoma), PC-3 (ATCC-CRL-1435, prostate adenocarcinoma), B16-F10 (ATCC-CRL-6322, murine melanoma), and UACC (human melanoma). The cells were donated by João Ernesto de Carvalho, DSc., of the Chemical, Biological, and Agricultural Pluridisciplinary Research Center (CPQBA) of the Universidade Estadual de Campinas. The non-neoplastic cell line NIH/3T3 (ATCC-CRL-1658, murine fibroblast) used in the assays was acquired from the Rio de Janeiro Cell Bank. All cell lines were cryopreserved in liquid nitrogen.The cells were thawed and cultured in sterile flasks with a 25 cm2 capacity containing RPMI 1640 medium and 1% penicillin–streptomycin, supplemented with 10% fetal bovine serum (FBS) (complete medium), and incubated at 37 °C in a humid atmosphere containing 5% CO2, until formation of a monolayer with at least 80% confluence. The cells were detached from the flasks by trypsinization and deposited into microplates at a density of 7500 cells per well.39
In triplicate, test samples at 0.25, 2.5, 25, and 250 μg mL−1 were added to the test plates in DMSO solution and diluted in complete medium (at the highest DMSO concentration used in the assay, of 0.25%, so as not to affect cell viability). The test plate (T) contained the blank for each sample concentration, the negative control (cells in culture medium), and the positive control (doxorubicin at 0.025, 0.25, 2.5, and 25 μg mL−1). After 48 h of sample incubation, a cytotoxicity test was performed after addition of sulforhodamine B (SRB).40
From the absorbance values, read at 540 nm on a microplate reader, growth percentages were calculated for each concentration using Microsoft Office Excel 2007 software.41 From the growth percentages in triplicate, means and standard deviations were calculated for each concentration (0.25, 2.5, 25, and 250 mg mL−1). Nonlinear regression was performed on these results using Microcal Origin, version 6.0, software to calculate relative potency values (GI50)—i.e., the concentrations required to inhibit cell growth by 50%.
3. Results and discussion
3.1. IR and NMR spectroscopy
Ozonolysis reaction times of 12, 24, and 36 h were employed for sunflower and baru oils (SO/BO-12, SO/BO-24 and SO/BO-36, respectively), and 12 and 24 h for flaxseed oil (FO-12 and FO-24). After 24 h of reaction, the product of flaxseed oil became highly viscous, preventing ozone gas from bubbling into the reaction medium. By increasing ozonation time, the fatty acid chain loses mobility, becoming more rigid, probably as a result of formation of three ozonides moieties linked by methylene groups from the triene present in the acyl groups of flaxseed oil. Formation of the three rings may cause changes in van der Waals-type intermolecular interactions taking place between the fatty acid chains.19,42
The chemical changes resulting from ozonolysis were analyzed by IR, 1H, and 13C NMR spectroscopy.
For all three oils, the FT-IR data revealed characteristic bands of double bonds at 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, stretching) and 3009 (C
C, stretching) and 3009 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, stretching) cm−1. Upon increasing reaction times, these bands diminished and a new band at 1106 cm−1 appeared, attributed to C–O stretching of ozonides. Aldehyde bands were absent from the IR spectra of the ozonized oils.5,19
C–H, stretching) cm−1. Upon increasing reaction times, these bands diminished and a new band at 1106 cm−1 appeared, attributed to C–O stretching of ozonides. Aldehyde bands were absent from the IR spectra of the ozonized oils.5,19
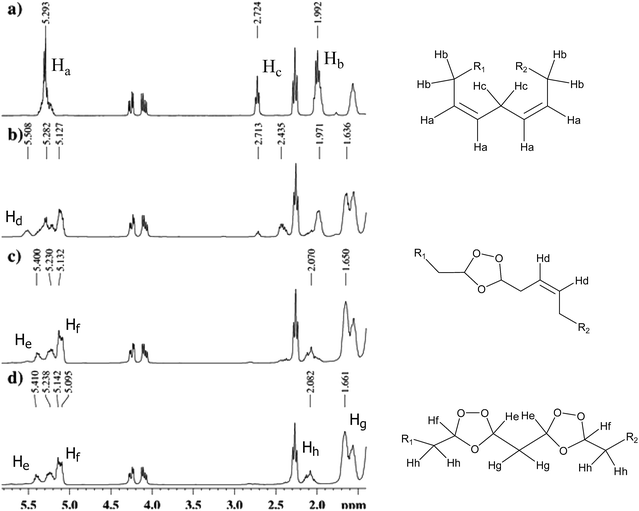
In the 1H NMR spectrum of sunflower oil before ozonolysis (SO-0, Fig. 1a), signals for olefinic protons (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) were observed in the region from 5.20 to 5.39 ppm. The signals at 1.99 and 2.72 ppm corresponded to methylene protons of the α-double bond (–CH2–CH
CH–) were observed in the region from 5.20 to 5.39 ppm. The signals at 1.99 and 2.72 ppm corresponded to methylene protons of the α-double bond (–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–) and the methylene group between the double bonds (–CH
CH–) and the methylene group between the double bonds (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), respectively. The double doublets at 4.10 and 4.26 ppm, attributed to methylene hydrogens of the groups at the sn-1 and 3 positions of the glycerol moiety, were preserved in the ozonated oil spectrum, indicating that no changes occurred in the glycerol moiety during ozonolysis.
CH–), respectively. The double doublets at 4.10 and 4.26 ppm, attributed to methylene hydrogens of the groups at the sn-1 and 3 positions of the glycerol moiety, were preserved in the ozonated oil spectrum, indicating that no changes occurred in the glycerol moiety during ozonolysis.
The 13C NMR spectrum exhibited two signals, at 172 and 173 ppm, for carbonyl carbons esters; sp2 carbons corresponding to unsaturation of fatty acids were identified in the signals located from between 127 and 130 ppm. Signals at 62 and 68 ppm corresponded to the carbons of glycerol, CH2, and CH, respectively, and those between 34 and 22 ppm to other carbons present in the structure.
After 24 h of reaction, the signals corresponding to olefinic protons at 5.29 ppm disappeared (Ha, Fig. 1a). Ozonides, the principal products of the reaction, showed signals at 5.14–5.09 ppm in the 1H NMR and 104 ppm in the 13C NMR spectra, and a multiplet at 5.45–5.35 ppm, which can be attributed to the hydrogen of ozonides linked by a methylene group (Hf and He, Fig. 1c and d).19 Aldehydes were formed at very low concentrations by an intermediate reaction (evidenced by a weak signal at 9.72 ppm), according to the mechanism proposed for ozonolysis.25
Fig. 1 shows the progress of ozonolysis in sunflower oil. Multiplets at 2.99 (Hb) and 5.29 (Ha) and a triplet at 2.72 (Hc) ppm, present in the 1H NMR spectrum of this oil prior to reaction, were absent from the spectra obtained after 24 and 36 h of ozonolysis. With the formation of ozonides, new signals appeared at 1.66 and 2.08 ppm (Hh and Hg), corresponding to methylene groups at the α position and between rings, respectively. The multiplet at 5.5 ppm (Hd Fig. 1b) in the spectrum of SO-12 and its absence from other spectra (SO-24 and SO-36) indicate formation of a homoallylic ozonide. In the reaction with linoleate, whose chain has two unconjugated double bonds, ozone initially reacts with one double bond, leading to formation of a homoallylic ozonide and shift of the signal for the remaining double bond hydrogens from 5.3 to 5.5 ppm.19
In the 1H NMR of FO-0, differences were observed in the chemical shifts of the methyl terminal group of linolenic acyl at 0.95 ppm and the methyl terminal group of other acyl groups at 0.85 ppm.43
The spectra of ozonated flaxseed and baru oils followed the same signal patterns observed for ozonated sunflower oil, indicating formation of ozonides. The signal at 5.5 ppm, attributed to olefinic protons (homoallylic ozonide), was observed in the 1H NMR spectrum of ozonated flaxseed oil (FO-24), but not in the spectra of ozonated sunflower and baru oils (SO-24 and BO-24, respectively).
The absence of signals in the region between 130–127 ppm and the presence of new signals between 104–100 ppm was related to ozonide carbons in the 13C spectrum of SO-24, indicating complete reaction of ozone with carbon–carbon double bonds.
The 13C NMR spectrum of flaxseed oil ozonated for 24 h showed signals at 136–120 ppm corresponding to sp2 carbons. For sunflower and baru oils, however, after 24 h all double bonds reacted with ozone to form ozonides.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. The spectrum of GEL-24 has an additional band (3446 cm−1) (Fig. 2) indicating the presence of hydroxyl groups, probably belonging to α-hydroxy-hydroperoxides, as well as free water from the reaction medium.
C double bonds. The spectrum of GEL-24 has an additional band (3446 cm−1) (Fig. 2) indicating the presence of hydroxyl groups, probably belonging to α-hydroxy-hydroperoxides, as well as free water from the reaction medium.
In the 1H NMR spectrum, a signal at 7.99 ppm indicated formation of α-hydroxy-hydroperoxides. Also, an aldehyde multiplet was found at 9.73 ppm (Fig. 3). Multiplets at 5.12 and 5.39 ppm were attributed to hydrogens in ozonides, as described earlier for the ozonated oils.
 | ||
| Fig. 3 Products formed from ozonolysis in the presence of water, and chemical shifts observed in the 1H NMR spectra for He and Hf. | ||
According to the mechanism proposed by Criegee, ozonolysis occurring in the presence of a protic solvent, such as water, leads to formation of α-hydroxy-hydroperoxides and aldehydes.25,26
3.2. Acid, iodine, and peroxide values
Oils contain a high proportion of unsaturated fatty acids, leading to a complex ozonated system. For all oils tested, acid values were found to increase with reaction time (Table 1), indicating that acids can be formed through decomposition of ozonides or directly during the reaction. After 24 h of reaction, the 1H NMR spectra of sunflower and baru oils showed no signals attributable to double bonds, as discussed earlier. Owing to saturation of the ozonated oil, ozone can react with aldehydes, forming carboxylic acids.44| Samplea | Acid value (mg of KOH g−1) | Iodine value (g of iodide (100 g)−1 of sample) | Peroxide value (meq kg−1) |
|---|---|---|---|
| a SO: sunflower oil; FO: flaxseed oil; BO: baru oil; T: reaction time, in hours.b Procedure adapted to determine the peroxide value of oleogel. | |||
| SO-0 | 0.20 ± 0.02 | 114.15 ± 1.53 | 20.06 ± 0.02 |
| SO-12 | 1.70 ± 0.05 | 39.24 ± 0.83 | 1761.87 ± 55.2 |
| SO-24 | 2.50 ± 0.06 | 4.46 ± 1.32 | 2065.63 ± 11.63 |
| SO-36 | 10.66 ± 0.02 | 0.49 ± 0.50 | 2151.49 ± 77.5 |
| FO-0 | 0.88 ± 0.04 | 175.14 ± 2.24 | 44.47 ± 3.76 |
| FO-12 | 2.13 ± 0.08 | 114.33 ± 3.18 | 1617.95 ± 2.01 |
| FO-24 | 4.25 ± 0.09 | 44.61 ± 3.18 | 2016.52 ± 58.3 |
| BO-0 | 0.32 ± 0.03 | 89.47 ± 0.49 | 15.58 ± 0.04 |
| BO-12 | 1.27 ± 0.03 | 37.78 ± 2.52 | 2324.41 ± 28.9 |
| BO-24 | 2.47 ± 0.09 | 0.71 ± 1.00 | 2619.82 ± 11.4 |
| BO-36 | 8.00 ± 0.01 | — | 1988.95 ± 18.6 |
| GEL-24 | 39.06 ± 0.94 | 7.50 ± 2.99 | 1933.11 ± 9.12b |
Of the samples investigated, oleogel exhibited the highest acidity index, which can be explained by the formation of other peroxidic compounds in the presence of water, such as α-hydroxy-hydroperoxides.
The iodine value is a measure of the total number of double bonds present in an oil sample.33 Ozone reacts with the double bonds of vegetable oils, leading to a rapid decrease in iodine value with increasing reaction time (Table 1). Given the concentration of unsaturated fatty acids, flaxseed oil exhibited the highest iodine value (175.14 g (100 g−1)), followed by sunflower oil (114.15 g (100 g)−1) and baru oil (89.47 g (100 g)−1). Baru oil has a high monounsaturated oleic acid content, and after 24 h of reaction all of its double bonds reacted with ozone (iodine value of BO-24: 0.71 g (100 g)−1), obviating the need to determine the iodine value at 36 h. Nonetheless, flaxseed oil (FO-24) showed an iodine value of 44.61 g (100 g)−1, indicating the presence of double bonds, as evidenced by the signals at 136–120 ppm in the 13C NMR spectra, as described earlier.
The results also showed an increase in peroxide values with longer reaction times. Determination of the amount of peroxide present in ozonated oils is crucial, since the ozonides formed are responsible for the biological activity of these compounds.45 Peroxide values are commonly determined by iodometric techniques, given the ability of peroxidic compounds to oxidize iodide to iodine. No reports are available on the mechanism of reaction of ozonides with potassium iodide. One possible mechanism is the decomposition of ozonide into hydroperoxide, which can be reduced by iodide, causing the release of iodine.35
The peroxide value obtained for GEL-24 was 1933.11 ± 9.12 meq kg−1, and a 1H NMR spectrum recorded after titration showed no signals corresponding to peroxidic compounds, indicating that all peroxide was oxidized by iodine to iodide.
3.3. Differential scanning calorimetry
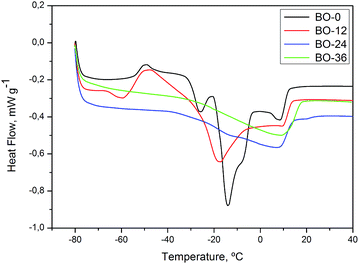
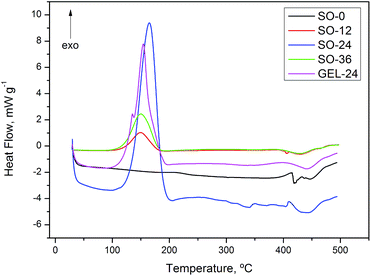
Samples of oils and ozonated oils were subjected to differential scanning calorimetry (DSC) analysis.At low temperatures, the DSC heat curve for SO-0, BO-0 and FO-0 (Fig. 4–6) shows endothermic transitions corresponding to triglyceride (TAG) melting. Generally, interpreting melting curves of oil samples is a laborious task, given the wide variety and complex nature of TAGs in vegetable oils.46 All samples of oils without ozonolysis (SO-0, FO-0 and BO-0) shows more than one endothermic transition to melting, suggesting polycrystalline state and the ozonated oil samples underwent endothermic transitions at higher temperatures than oils without ozonolysis.
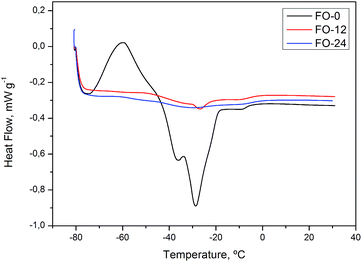
Glass transition at about −56 °C was observed in the heat curve for GEL-24 (Fig. 4), a characteristic feature of polymeric materials or substances that undergo non-crystalline solidification. Glass transition temperature is strongly dependent on polymer chain chemical composition and intermolecular interactions, such as hydrogen bonds. Ozonolysis of sunflower oil was performed in the presence of water, producing compounds capable of interacting with each other and with water molecules by way of hydrogen bonds.47,48 Glass transition was followed by an endothermic peak corresponding to melting temperatures from −30 to 12 °C and a fusion heat (ΔH) of 19.78 J g−1.
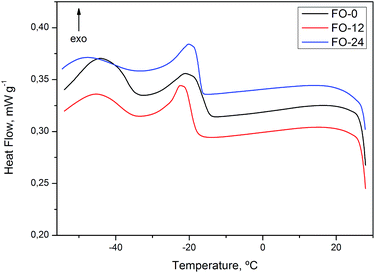
DSC heat curve of flaxseed oil FO-0 (Fig. 5) shows exothermic transition from −73 to −61 °C indicating a possible phase transition. At curves of FO-12 and FO-24, transitions were not well defined, being necessary to perform further analysis using heating rate lower than 20 °C min−1. Baru oils, BO-0, 12, 24 and 36, exhibited similar thermal behavior to sunflower oils (Fig. 6).
Exothermic transitions can be observed in the cooling curve for DSC, corresponding to crystallization of fatty acids, while sunflower oil (SO-0) exhibited transition characteristic of unsaturated acids, at −20.80 °C (Fig. 7). Ozonolysis leads to a decrease in unsaturated acids, as discussed earlier.
As expected, transitions at −3 and −15 °C, observed in ozonated samples, indicate a reaction of ozone with a double bonds and similar thermal behavior of saturated fatty acids. For oil samples with high degrees of saturation, such as SO-24 (iodine value: 4.46 ± 1.32 mg KOH g−1) and GEL-24 (iodine value: 7.50 ± 2.99 mg KOH g−1), melting and crystallization profiles involved higher temperature than for oils with high degrees of unsaturation.46
Cooling DSC curves for flaxseed (FO-0, 12 and 24) and baru (BO-0, 12, 24 and 36) oils exhibited similar thermal behavior to sunflower oils (Fig. 8 and 9).
On the DSC thermal degradation curves for SO-12, 24, 36 and GEL-24 (Fig. 10), exothermic peaks were attributed to degradation followed by oxidative decomposition of ozonides at about 150 °C. Oxidative decomposition was probably caused by oxygen released from ozonide degradation. This hypothesis is in agreement with the curve for non-ozonated sunflower oil (SO-0), devoid of exothermic peaks at this temperature. The thermal analysis for ozonated flaxseed and baru oils exhibited exothermic peaks related to decomposition at similar temperature (Fig. 11 and 12).
Cataldo28 have made a thermochemical study by DSC and reported that ethyl oleate ozonide decomposes at 155 °C. The decomposition temperature of ozonides formed in ethyl oleate is coincident with that found for ozonated sunflower, flaxseed and baru oils (Table 2). Thermal decomposition enthalpy is proportional to the degree of ozonization reached and the increase in the amount of ozonides present in ozonated oils is manifested by an increasing amount of released energy (ΔH decomposition) during the thermal decomposition.
| Sample | Decomposition temperature (°C) | Decomposition enthalpy (J g−1) |
|---|---|---|
| SO-0 | n.a. | 0 |
| SO-12 | 150.3 | 307.0 |
| SO-24 | 165.0 | 1368.0 |
| SO-36 | 150.7 | 646.4 |
| GEL-24 | 154.0 | 849.3 |
| FO-0 | n.a. | 0 |
| FO-12 | 147.6 | 140.6 |
| FO-24 | 146.3 | 307.0 |
| BO-0 | n.a. | 0 |
| BO-12 | 153.3 | 194.8 |
| BO-24 | 153.1 | 335.8 |
| BO-36 | 152.8 | 537.3 |
Flaxseed oil have higher degree of unsaturation in comparison to sunflower and baru oils, as measured by the iodine value, ∼175, 114 and 89 g of iodide (100 g)−1 of sample, respectively. However, flaxseed oil presented lower decomposition enthalpy than sunflower and baru oil. It can be explained because the efficiency of ozonolysis reaction is lowest in flaxseed oil, probably due to highly viscosity, preventing ozone gas from bubbling into the reaction medium.
3.4. Antimicrobial activity
In 90% of cases, skin infections are caused by S. aureus and Streptococcus pyogenes, while P. aeruginosa and E. coli may participate as secondary agents.49,50 Infection is one of the main factors affecting the healing process, because, while cells are fighting bacteria, the inflammatory stage is extended, inhibiting the ability of fibroblasts to produce collagen.51Emergence of bacterial strains with increasing levels of resistance to antimicrobials has become a cause for concern, particularly in immunocompromised patients with chronic wounds who are undergoing invasive procedures in hospital, which expose them to further infection. Nosocomial infections represent a challenge to clinical practice, extending hospital stays, increasing morbidity and mortality rates and elevating hospitalization costs.52,53
Ozonated sunflower oils have a broad antimicrobial spectrum, exhibiting inhibitory and lethal activity against Gram-positive and Gram-negative bacteria, including antibiotic-resistant clinical strains.45,50 Sechi et al. evaluated the antimicrobial activity of ozonated sunflower oil (Oleozon®) against standard strains and found MIC values of 9.5 mg mL−1 for E. faecalis and S. aureus and 4.75 mg mL−1 for E. coli and P. aeruginosa.50 The ozonated oil affected bacterial cell permeability by promoting a loss of intracellular K+ ion content and induced a reduction of cytoplasmic contents in S. aureus.54
Using the agar dilution method (NCCLS, 1993), ozonated sunflower, flaxseed, and baru oils along with oleogel were tested against Gram-negative P. aeruginosa (ATCC 27853) and E. coli (ATCC 25922) and Gram-positive E. faecalis (ATCC 29212 and a clinical strain resistant to penicillin G, ampicillin, ciprofloxacin, moxifloxacin, norfloxacin, erythromycin, clindamycin, teicoplanin, and vancomycin) and S. aureus (ATCC 25923 and a clinical strain resistant to benzylpenicillin, oxacillin, gentamicin, ciprofloxacin, moxifloxacin, norfloxacin, erythromycin, clindamycin, rifampicin, and sulfamethoxazole trimethoprim). Their respective MIC values are shown in Table 3.
| Sample | MIC (mg mL−1) | |||||
|---|---|---|---|---|---|---|
| P. aeruginosa (ATCC) | E. faecalis (ATCC) | E. faecalis (clinical strain) | S. aureus (ATCC) | S. aureus (clinical strain) | E. coli (ATCC) | |
| a n.a: not active at the concentrations tested. | ||||||
| SO-12 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| SO-24 | n.a. | 10 | 10 | n.a. | 10 | n.a. |
| SO-36 | n.a. | n.a. | n.a. | 7 | 7 | 7 |
| FO-12 | n.a. | n.a. | n.a. | 10 | 10 | 10 |
| FO-24 | n.a. | 5 | 5 | 5 | ≤3 | 10 |
| BO-12 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| BO-24 | n.a. | n.a. | n.a. | n.a. | 7 | 7 |
| BO-36 | n.a. | n.a. | n.a. | 10 | 10 | 10 |
| GEL-24 | 7 | 7 | 7 | ≤3 | ≤3 | ≤3 |
In general, ozonated oils proved more active against both standard and clinical strains of Gram-positive E. faecalis and S. aureus. Tested against both standard and clinical strains of S. aureus and E. coli, flaxseed oil ozonated for 12 h (FO-12) exhibited MIC = 10 mg mL−1, while sunflower and baru oils ozonated for the same times (SO-12 and BO-12) were inactive at the concentrations tested. Flaxseed oil ozonated for 24 h (FO-24) proved remarkably active against all Gram-positive strains tested, with MIC = 5 mg mL−1 for E. faecalis (clinical strain) and MIC ≤ 3 mg mL−1 for S. aureus (clinical strain). Other oils ozonated for longer (24 and 36 h) were also active, with MIC values of 7 to 10 mg mL−1 against at least one strain. These results showed flaxseed and sunflower oils to be more active than baru oil, a feature possibly related to their high content of peroxidic compounds.
Oleogel was active against all strains tested, including P. aeruginosa (MIC = 7 mg mL−1), the resistant clinical strains of E. faecalis (MIC = 7 mg mL−1) and S. aureus (MIC ≤ 3 mg mL−1). Activity against resistant bacterial strains, observed for many of the oils tested, is a noteworthy finding, particularly in the case of S. aureus, a major problem in wound treatment.
The MIC values obtained for ozonated oils, in the range of milligrams per milliliters, may seem high when compared with the concentration of antibiotics, in the range of mere micrograms per milliliter, required for inhibition of microbial growth. However, vegetable oils are complex mixtures of antioxidants and high-molecular-weight triacylglycerols, acting as a matrix capable of releasing active oxygen from ozonides, which have antimicrobial activity. The MIC values obtained for oleogel and ozonated oils (molecular weight of around 1096.54 g mol−1) can be considered excellent, given that antimicrobial agents, when tested, are employed in pure form and not as complex mixtures.55
3.5. Cytotoxicity and antitumor activity
Although ozonated oils have been used in many countries in the treatment of wounds and skin ulcers, reports on their cytotoxicity are scarce.In the present study, the ozonated oils that exhibited better results on the antimicrobial test were further investigated for cytotoxicity against NIH/3T3 fibroblasts (Table 4), but proved non-toxic when compared with doxorubicina desirable trait for the safe use of ozonated oils in patients.
| Cell line | GI50 (μg mL−1) | |||||
|---|---|---|---|---|---|---|
| SO-24 | SO-36 | GEL-24 | FO-12 | FO-24 | Doxorubicin | |
| a Non-neoplastic. | ||||||
| 786-0 | 57.08 | 61.21 | 47.16 | >250 | >250 | 0.19 |
| HT-29 | 31.16 | 50.77 | 53.94 | >250 | >250 | 0.28 |
| MCF7 | 24.31 | 27.33 | 25.54 | 40.24 | 39.19 | 0.21 |
| PC-03 | 26.22 | 36.42 | 30.93 | >250 | 201.48 | 0.27 |
| B16-F10 | 22.67 | 28.97 | 60.35 | 25.25 | 234.14 | 0.02 |
| NIH/3T3a | 49.09 | 74.50 | 65.48 | 61.29 | 62.80 | 2.3 |
Ozone selectively inhibits cell growth in human lung, breast, and uterine tumors, compared with non-cancerous human lung diploid fibroblasts.56
Important cytotoxic properties of ozonides and other endoperoxides have been reported. These compounds probably generate oxygen reactive species (ROS) that cause apoptosis by induced oxidative stress.57 Arakawa et al.58 reported that ozonide (cyclic peroxide) exhibits multiantiangiogenic and potent antitumor activity in vitro and in vivo against human fibrosarcoma HT-1080 cells. However, no data were found regarding the antitumor properties of ozonated oils.
In the present study, the antitumor potential of ozonated oils was investigated using five cancer cell lines: 786-0 (ATCC-CRL-1932, renal adenocarcinoma) HT-29 (ATCC-HTB-38, colon adenocarcinoma), MFC-7 (ATCC-HTB-22, breast adenocarcinoma), PC-3 (ATCC-CRL-1435, prostate adenocarcinoma), and B16-F10 (ATCC-CRL-6322, murine melanoma). The GI50 values obtained are shown in Table 4. Ozonated oils with GI50 < 30 μg mL−1 were considered potentially active.59
The SO-24 sample of ozonated sunflower oil proved active against MCF7, PC-03, and B16-F10 cells. SO-36, GEL-24, FO-12, and FO-24 were active against MCF7 and B16-F10 cells.
4. Conclusion
Our study report the detailed chemical characterization of ozonated vegetable oils and oleogel, including an investigation of their differential scanning calorimetry behavior. Baru, one of the sources of these materials, is an important species from the Brazilian Cerrado biome.Ozonated oils showed antimicrobial activity against Gram-positive and Gram-negative bacteria. Oleogel, a novel product, proved the most active against multiresistant clinical strains of E. faecalis and S. aureus.
Ozonated oils and oleogel were potentially active against neoplastic cell lines and showed no cytotoxic activity when tested against NIH/3T3 murine fibroblast cells a desirable trait for the safe use of these products in patients with chronic wounds and other dermatological conditions, where minimal toxicity to normal cells is required.
Acknowledgements
The authors thank FUNDECT-MS and CNPq for their financial support. We are also grateful to the referees for their constructive advices to improve this paper.References
- G. Valacchi, V. Fortino and V. Bocci, Br. J. Dermatol., 2005, 153, 1096 CrossRef CAS PubMed.
- G. Valacchi, I. Zanardi, Y. Lim, G. Belmonte, C. Miracco, C. Sticozzi, V. Bocci and V. Travagli, Int. J. Pharm., 2013, 458, 65 CrossRef CAS PubMed.
- G. Martínez-Sánchez, L. R. G. Perez-Davison and R. H. Delaporte, Revista Española de Ozonoterapia, 2012, 2, 121 Search PubMed.
- V. Bocci, in Ozone: a New Medical Drug, Springer, 1st edn, 2005, p. 315 Search PubMed.
- I. Zanardi, V. Travagli, A. Gabbrielli, L. Chiasserine and V. Bocci, Lipids, 2008, 43, 877 CrossRef CAS PubMed.
- M. F. Díaz, R. Hernández, G. Martínez, G. Vidal, M. Gómez, H. Fernández and R. Garcés, J. Braz. Chem. Soc., 2006, 17, 403 CrossRef.
- L. V. Guerrer, K. C. Cunha, M. C. L. Nogueira, C. C. Cardoso, M. M. C. N. Soares and M. T. G. Almeida, Braz. J. Microbiol., 2012, 43, 1315 CrossRef CAS.
- M. Montevecchi, A. Dorigo, M. Cricca and L. Checchi, New Microbiol., 2013, 36, 289 CAS.
- F. V. Daud, S. M. Y. Ueda, A. Navarini and L. M. J. Mímica, Braz. J. Microbiol., 2011, 42, 274 CrossRef PubMed.
- L. A. Sechi, I. Lezcano, N. Nunez, M. Espim, I. Dupre, A. Pinna, P. Molicotti, G. Fadda and S. Zanetti, J. Appl. Microbiol., 2001, 90, 279 CrossRef CAS.
- G. Valacchi, Y. Lim, G. Belmonte, C. Miracco, I. Zanardi, V. Bocci and V. Travagli, Wound Repair Regen., 2011, 19, 107 CrossRef PubMed.
- S. A. Pai, S. A. Gagangras, S. S. Kulkarni and A. S. Majumdar, Indian J. Pharm. Sci., 2014, 76, 87 CAS.
- J. John, M. Bhattacharya and P. C. Raynor, Colloids Surf., A, 2004, 237, 141 CrossRef CAS PubMed.
- M. F. Díaz, J. A. G. Sazatornil, O. Ledea, F. Hernández, M. Alaiz and R. Garcés, Ozone: Sci. Eng., 2005, 27, 247 CrossRef PubMed.
- J. Sadowska, B. Johansson, E. Johannessen, R. Friman, L. Broniarz-Press and J. B. Rosenholm, Chem. Phys. Lipids, 2008, 151, 85 CrossRef CAS PubMed.
- A. Sega, I. Zanardi, L. Chiasserini, A. Gabbrielli, V. Bocci and V. Travagli, Chem. Phys. Lipids, 2010, 163, 148 CrossRef CAS PubMed.
- M. F. Díaz, Y. Sánchez, M. Gómez, F. Hernández, M. C. C. Veloso, P. A. P. Pereira, A. S. Mangrich and J. B. de Andrade, Grasas Aceites, 2012, 63, 466 CrossRef.
- M. Cirlini, A. Caligiani, G. Palla, A. de Ascentiis and P. Tortini, Ozone: Sci. Eng., 2012, 34, 293 CrossRef CAS PubMed.
- P. Guerra-Blanco, T. Poznyak, I. Chairez and M. Brito-Arias, Eur. J. Lipid Sci. Technol., 2015, 117, 988 CrossRef CAS PubMed.
- I. F. Torres, V. C. Piñol, E. S. Urrutia and M. G. Regueiferos, Ozone: Sci. Eng., 2006, 28, 1 CrossRef PubMed.
- M. F. Díaz, F. Hernández, N. Núñez, D. Quincose and W. Díaz, Ozone: Sci. Eng., 2005, 27, 153 CrossRef PubMed.
- J. Zabicky and M. Mhasalkar, J. Am. Oil Chem. Soc., 1986, 63, 1547 CrossRef CAS.
- K. Skalska, S. Ledakowicz, J. Perkowski and B. Sencio, Ozone: Sci. Eng., 2009, 31, 232 CrossRef CAS PubMed.
- M. A. Khadre and A. E. Yousef, Int. J. Food Microbiol., 2001, 71, 131 CrossRef CAS.
- R. Criegee, Angew. Chem., Int. Ed., 1975, 14, 745 CrossRef PubMed.
- N. R. Almeida, A. Beatriz, A. C. Micheletti and E. J. Arruda, Orbital: Electron. J. Chem., 2012, 4, 313, DOI:10.17807/orbital.v4i4.467.
- N. U. Soriano, V. P. Migo and M. Matsumura, Electrochim. Acta, 2005, 50, 1131 CrossRef CAS PubMed.
- F. Cataldo, Chem. Phys. Lipids, 2013, 175, 41 CrossRef PubMed.
- F. D. Gunstone, J. L. Harwood and F. B. Padley, The Lipid Handbook. Occurrence and Characteristics of Oils and Fats, Chapman & Hall, London, 2nd edn, 1994 Search PubMed.
- S. M. Sano, J. F. Ribeiro and M. A. Brito, Baru: biologia e uso. Embrapa CPAC: Platina, 2004, p. 116.
- E. Takemoto, I. A. Okada, M. L. Garbelotti, M. Tavares and S. Aued-Pimentel, Rev. Inst. Adolfo Lutz, 2001, 60, 113 CAS.
- European Pharmacopoeia. Acid value, Council of Europe, 5th edn, Strasbourg Cedex, France, 2004, pp. 127.
- European Pharmacopoeia. Iodine value, Council of Europe, 5th edn, Strasbourg Cedex, France, 2004, pp. 127–128.
- Instituto Adolfo Lutz, Normas analíticas do Instituto Adolfo Lutz. v.1: Métodos Químicos e Físicos para Análise de Alimentos, 3rd edn, 1985, pp. 245–246 Search PubMed.
- G. M. Tellez, O. L. Lozano and M. F. D. Gómez, Ozone: Sci. Eng., 2006, 28, 181 CrossRef CAS PubMed.
- AOCS, Peroxide value. AOCS official method, in Official Methods and Recommended Practices of AOCS, ed. D. Firestone, American Oil Chemists’ Society, Champaign, IL, 5th edn, 1998, p. 8 Search PubMed.
- E. Richaud, F. farcas, B. Fayolle, L. Audouin and J. Verdu, Polym. Test., 2006, 25, 829 CrossRef CAS PubMed.
- NCCLS, National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, M7-A3, 3rd edn, 1993, p. 13 Search PubMed.
- I. R. Freshney, Culture of Animal Cells: a Manual of Basic Technique, Wiley-Liss, New York, 5th edn, 2005 Search PubMed.
- P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. Mcmahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82, 1107 CrossRef CAS PubMed.
- A. Monks, D. Scudiero, P. Skehan, R. Shoemaker, K. Pau, D. Vistica, H. Curtis, J. Langley, P. Cronise, A. Vaigro-Wolff, M. Gray-Goodrich, H. Campbell, J. Mayo and M. Boyd, J. Natl. Cancer Inst., 1991, 83, 757 CrossRef CAS PubMed.
- M. G. Constantino and G. V. J. Silva, Fundamentos de Química, Atheneu, 1st edn, 2014, p. 121 Search PubMed.
- M. D. Guillén and A. Ruiz, Trends Food Sci. Technol., 2001, 12, 328 CrossRef.
- J. Santrock, R. A. Gorski and J. F. O'gara, Chem. Res. Toxicol., 1992, 5, 134 CrossRef CAS.
- M. Díaz, I. Lezcano, J. Molerio and F. Hernández, Ozone: Sci. Eng., 2001, 23, 35 CrossRef PubMed.
- C. P. Tan and Y. B. Che Man, J. Am. Oil Chem. Soc., 2000, 77, 2 CrossRef.
- Q. H. Zhou, M. Li, P. Yang and Y. Gu, Macromol. Theory Simul., 2013, 22, 107–114 CrossRef CAS PubMed.
- S. B. Yamaki, A. G. Pedroso and T. D. Z. Atvars, Quim. Nova, 2002, 25, 330 CrossRef CAS.
- R. Alvarez, S. Menendez, M. Peguera and J. Turrent, Treatment of primary pioderma with ozonized sunflower oil, in Second International Symposium on Ozone Applications, Havana, Cuba, 24–26, March 1997, p. 76 Search PubMed.
- L. A. Sechi, I. Lezcano, N. Nuñez, M. Espim, I. Dupre and A. Pinna, J. Appl. Microbiol., 2001, 90, 279 CrossRef CAS.
- S. H. Mandelbaum, E. P. Di Santis and M. H. S. Mandelbaum, An. Bras. Dermatol., 2003, 78, 393 Search PubMed.
- L. S. Berquó, A. J. D. Barros, R. C. Lima and A. D. Bertoldi, Rev. Saude Publica, 2004, 38, 239 CrossRef PubMed.
- D. Andrade, V. C. Leopoldo and V. J. Haas, Revista Brasileira de Terapia Intensiva, 2006, 18, 27 CrossRef PubMed.
- V. Curtiellas, O. Ledea, S. Rodríguez, O. Ancheta, M. Echevarría, E. Sánchez and I. Fernández, Rev. CENIC, Cienc. Biol., 2008, 39, 128 Search PubMed.
- K. L. Rodrigues, C. C. Cardoso, L. R. Caputo, J. C. T. Carvalho, J. E. Fiorini and J. M. Schneedorf, Inflammopharmacology, 2004, 12, 261 CrossRef CAS PubMed.
- F. Sweet, M. S. Kao, S. C. Lee, W. L. Hagar and W. E. Sweet, Science, 1980, 209, 931 CAS.
- A. Niesen, A. Barthel, R. Kluge, A. Köwitzsch, D. Ströhl, S. Schwars and R. Csuk, Arch. Pharm. Chem. Life Sci., 2009, 342, 569 CrossRef CAS PubMed.
- K. Arakawa, Y. Endo, M. Kimura, T. Yoshida, T. Kitaoka, T. Inakazu, Y. Nonami, M. Abe, A. Masuyama, M. Nojima and T. Sasaki, Int. J. Cancer, 2002, 100, 220 CrossRef CAS PubMed.
- A. Itharat, P. J. Houghton, E. Eno-Amooquaye, P. J. Burke, J. H. Sampson and A. Raman, J. Ethnopharmacol., 2004, 90, 33 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02798e |
| This journal is © The Royal Society of Chemistry 2015 |