Controlled synthesis and enhanced electrochemical performance of Prussian blue analogue-derived hollow FeCo2O4 nanospheres as lithium-ion battery anodes†
Lei Liua,
Zhongbo Hua,
Limei Sunb,
Gui Gaoa and
Xiangfeng Liu*a
aCollege of Materials Science and Opto-Electronic Technology, University of Chinese Academy of Sciences, Beijing 100049, China. E-mail: liuxf@ucas.ac.cn
bDepartment of Nuclear Physics, China Institute of Atomic Energy, Beijing 102413, China
First published on 31st March 2015
Abstract
Porous metal oxides have attracted great interest as anode materials for lithium ion batteries owing to their improved electrochemical properties. In this study, we propose a Prussian blue analogue (PBA)-derived strategy to successfully prepare hollow porous FexCo3−xO4 (FCO) with controlled morphologies (nanospheres and nanocubes) using surfactants as “soft templates”. In comparison with FCO nanocubes (FCO-NCs) and FCO nanoparticles (FCO-NPs), FCO spheres (FCO-NSs) show a much better cycling stability and rate capability as an anode material for lithium ion batteries. The cycling capacity of FCO-NSs at the 50th cycle has been largely enhanced to 1060 mA h g−1 from only 721 (FCO-NCs) and 389 mA h g−1 (FCO-NPs). The capacity of FCO-NSs at a current density of 1000 mA g−1 has been considerably improved to 823 mA h g−1 from 504 and 152 mA h g−1 for FCO-NCs and FCO-NPs, respectively, indicating a much better rate capability. The greatly enhanced cycling stability and rate capability can be largely attributed to the hollow porous structure of FCO-NSs with a wider pore distribution, a slightly higher Co content (compared to FCO-NCs) and higher mechanical strength, which facilitates Li+ and electron diffusion and migration.
1. Introduction
Lithium ion batteries (LIBs) are being widely used in consumable electronic devices. Because of the upgrade demands of these electronics and the enormous market prospects for electric vehicles, extensive efforts have been made to develop high performance lithium ion batteries with higher capacities, rate capabilities and safety.1–7 Transition metal oxides have attracted considerable attention because of their much higher capacity than that of commercial graphite anodes. Co3O4 with a spinel structure has been considered to be a promising anode material for LIBs owing to its high specific capacity (about 890 mA h g−1),8–11 but the high cost and the potential environmental pollution from the Co source restrict the wide application of Co3O4 in LIBs.Recently, some binary transition metal oxides (AB2O4, A = Mn, Fe, Co, Ni, Cu, Zn; B = Mn, Fe, Co, Ni, Cu, Sn; A ≠ B), which also crystallize in a spinel structure, have attracted much interest as lithium ion battery anodes because of their high theoretical capacity (∼900 mA h g−1) and lower cost in comparison with Co3O4.12–22 But due to the limited diffusion rate of lithium ions in the bulk material, their low conductivity and the large volume change during the lithium insertion and extraction processes, these metal oxide anodes usually show very poor cycling performances and rate capabilities, which restrict their practical application. Some approaches such as the fabrication of oxide/carbon nanocomposites23–26 or oxide arrays loaded on conductive substrates9,27,28 could reduce the internal resistance and provide structural supports to improve their electrochemical performance. Another way to improve the conductivity of a material is by electrospinning to obtain nanofibers.29,30 For instance, Teh et al.29 obtained an electrospun ZnFe2O4 nanoweb composed of one-dimensional fibers with a reversible capacity of 733 mA h g−1 up to 30 cycles at 60 mA g−1. Hollow/porous nanostructure constructions may provide a shorter migration pathway for lithium ions, larger electrochemically-active areas and a buffer space for alleviating the strain during charging/discharging. This strategy has been extensively applied to improve the electrochemical properties of binary transition metal oxides.31–37 For examples, Zhang et al.31 greatly improved the cycling performance of ZnMn2O4 by making ball-in-ball hollow microspheres with a reversible capacity of 750 mA h g−1 after 120 cycles at 400 mA g−1. Li et al.32 studied the electrochemical properties of CoFe2O4 with a three-dimensional ordered macroporous structure, which exhibited a capacity of 702 mA h g−1 after 30 cycles at a current density of 0.2 mA cm−2. However, until now, only a few studies have been reported on FeCo2O4 as an anode material for lithium ion batteries. Mohamed et al.38 have studied the electrochemical performance of FeCo2O4 nanoflake arrays on nickel films as electrodes for both Li-ion batteries and supercapacitors. Sharma et al.21 studied the electrochemical properties of FeCo2O4 and MgCo2O4 nanoparticles as anode materials for Li-ion batteries, and their results exhibit that the FeCo2O4 nanoparticles showed a discharge capacity of 752 mA h g−1 after 50 cycles at 60 mA g−1, much higher than that of MgCo2O4 (102 mA h g−1).
Metal–organic frameworks (MOFs) represent a kind of promising functional material because their structures can be flexibly tuned with abundant functions.39,40 When calcinated, MOFs may release gases and generate hollow porous structures.41,42 Thus, MOFs have great potential to be used for the synthesis of some new anode materials with hollow or porous structures. For example, ZnxCo3−xO4 hollow polyhedra have been synthesized using Zn–Co-ZIFs as the precursors.43 Prussian blue is a safe material with low economic and environmental cost. Zhang et al. have obtained Fe2O3 microboxes with hierarchical shell structures by annealing Prussian blue (PB) microcubes.44 These microboxes showed an improved cycling performance due to their hollow porous structure. Several lithium ion battery anode materials derived from Prussian blue or its analogues have also been listed in Table 1. More efforts are still needed to develop novel synthesis procedures to control the morphology and improve the performance at higher current densities.
| Samples | Capacities (mA h g−1) | Rate capacity (mA h g−1) | Ref. |
|---|---|---|---|
| FeCo2O4 nanospheres | 1060 (100 mA g−1; 50th) | 823 (1 A g−1) | This work |
| Fe1.2Co1.8O4 nanocubes | 721 (100 mA g−1; 50th) | 504 (1 A g−1) | This work |
| Co3O4 nanocages | 970 (50 mA g−1; 30th) | 487 (1 A g−1) | 49 |
| Fe2O3 microboxes | 945 (200 mA g−1; 30th) | >500 (1 A g−1) | 44 |
| Foam-like MnxCo3−xO4 | 730 (200 mA g−1; 30th) | 668 (800 mA g−1) | 50 |
| Fe2O3/Co3O4 hollow microcubes | 500 (100 mA g−1; 50th) | 272 (800 mA g−1) | 51 |
| Mn1.8Fe1.2O4 nanocubes | 827 (200 mA g−1; 60th) | 478 (800 mA g−1) | 52 |
| Fe2O3@NiCo2O4 porous nanocages | 1000 (200 mA g−1; 100th) | 662 (1 A g−1) | 53 |
In this study, we propose a novel method to control the morphologies of FexCo3−xO4 samples using surfactants as “soft templates” and to improve their electrochemical performance as anodes for lithium ion batteries. To the best of our knowledge, it is the first time that Fe1.2Co1.8O4 nanoparticles (FCO-NPs), Fe1.2Co1.8O4 nanocubes (FCO-NCs) and FeCo2O4 hollow nanospheres (FCO-NSs) have been directly prepared by thermal decomposition of shape-controlled Prussian blue analogue (denoted as PBA) precursors. Afterwards, the effects of the morphology of the obtained products on the electrochemical performances were investigated. Both the cycling performance and the rate capability of FCO-NSs are significantly improved and much better than those of the FCO-NPs and FCO-NCs. The reversible capacities of the FCO-NP, FCO-NC and FCO-NS samples at the 50th cycle were 389, 721 and 1060 mA h g−1, respectively. And compared to the FCO-NPs, the capacities of the FCO-NCs and FCO-NSs at a current density of 1000 mA g−1 were also obviously enhanced to 504 and 823 mA h g−1, respectively, which might be largely attributed to the hollow porous structure of the nanospheres and nanocubes. The electrochemical performance of the FCO-NSs was even better than that of the FCO-NCs, which may be due to the wider pore size distribution and the slightly higher Co content of the FCO-NSs than that of the FCO-NCs (Fe1.2Co1.8O4).
2. Materials and methods
2.1 Chemicals
Co(NO3)2·6H2O, polyvinylpyrrolidone (PVP, K30), sodium dodecyl sulfate (SDS), sodium dodecyl benzene sulfonate (SDBS), and K3[Fe(CN)6] were analytical grade and used without further purification.2.2 Synthesis of FCO-NPs, FCO-NCs and FCO-NSs
In a typical procedure for synthesizing the FCO-NPs, 3 mmol of Co(NO3)2·6H2O and 10 g of polyvinylpyrrolidone were dissolved in 300 mL of deionized water by magnetic stirring at room temperature. Then, 200 mL of a 10 mmol L−1 K3[Fe(CN)6] solution was added drop by drop. The mixture was continually stirred for 4 hours. The brown precipitate was isolated by centrifugation and washed a few times using distilled water and absolute ethanol. Finally, the product was dried in an electrical blowing oven at 80 °C for 12 h. The dried powder was calcinated in an electric furnace in an air atmosphere at 500 °C for 3 hours. For the synthesis of the FCO-NCs, 10 g of sodium dodecyl sulfate (SDS) was used instead of PVP. The other reagents and reaction conditions were the same as those for synthesizing the FCO-NPs. For the synthesis of the FCO-NSs, 10 g of sodium dodecyl benzene sulfonate (SDBS) was used instead of SDS. The other reagents and reaction conditions were the same as those for the synthesis of the FCO-NCs.2.3 Characterization
XRD patterns were obtained using a PERSEE XD-3 X-ray diffractometer with CuKα radiation (λ = 1.5418 Å). The particle morphology was characterized using a field-emission scanning electron microscope (FESEM, Hitachi S4800) and a high resolution transmission electron microscope (HR-TEM, FEI Tecnai G2 F20). The BET specific surface areas of the products were calculated from the results of nitrogen physisorption at 77 K using a Micromeritics ASAP 2020 equipment.2.4 Electrochemical measurements
The electrochemical performance versus Li was measured using CR2016 coin cells. The working electrode consisted of cobalt ferrite nanoparticles (70 wt%), carbon black (20 wt%) and polyvinylidene fluoride (PVDF) (10 wt%). After the slurry was milled in an agate mortar for 30 min, it was coated onto Cu foil and dried at 80 °C for 12 h. The separator was a Celgard 2400 microporous membrane, and the electrolyte was a 1 mol L−1 solution of LiPF6 in ethylene carbonate/dimethyl carbonate (EC/DMC) with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The coin cells were assembled in an argon-filled glove box (Etelux, LAB2000) with Li metal as an anode. Galvanostatic discharge/charge tests were performed using a battery cycler in the voltage range of 0.01–3.0 V (vs. Li+/Li) at room temperature. The AC impedance spectrum was measured using an electrochemical workstation (PGSTAT 302N, Metrohm-Autolab).
1. The coin cells were assembled in an argon-filled glove box (Etelux, LAB2000) with Li metal as an anode. Galvanostatic discharge/charge tests were performed using a battery cycler in the voltage range of 0.01–3.0 V (vs. Li+/Li) at room temperature. The AC impedance spectrum was measured using an electrochemical workstation (PGSTAT 302N, Metrohm-Autolab).
3. Results and discussion
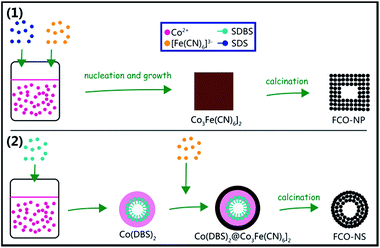
The procedure of synthesizing FCO from PBA includes two steps. For the FCO-NSs, the first step in aqueous solution is the formation of Co(DBS)2/Co3[Fe(CN)6]2·nH2O hybrids as precursors, and the second step is calcination in air to obtain FCO-NSs. For the FCO-NCs and FCO-NPs, the obtained precursors during co-precipitation were Co3[Fe(CN)6]2·nH2O. Fig. 1(a) shows the XRD patterns of the precursors synthesized using different surfactants and iron sources respectively, which agree well with the standard Joint Committee on Powder Diffraction Standards (JCPDS) card no. 46-0907. Very sharp peaks could be observed and no impurity peaks were detected. These patterns indicate that the three samples contain a single face-centered cubic phase of potassium hexacyanoferrate, and that these samples all have fine crystallinity. Co(DBS)2 micelles in the precursor of the FCO-NSs were not detected, which may be due to non-crystallinity. The crystallographic structures of the samples after calcination in air were analyzed using X-ray powder diffraction (XRD) as is shown in Fig. 1(b). All of the peaks of the samples are very similar to spinel Co3O4 (JCPDS card no. 43-1003, lattice constant α = 8.084 Å), but do not correspond well to spinel CoFe2O4 (JCPDS card no. 22-1086, lattice constant α = 8.392 Å). This may be due to the relatively large difference in the lattice constant between the FCO samples and CoFe2O4. These results also indicated that both the FCO-NSs and FCO-NPs were single-phase materials, rather than CoFe2O4–Co3O4 hybrids that possessed coexisting XRD spectra.39 To further confirm the elemental ratio of Fe/Co in the FCO-NC and FCO-NS samples, energy-dispersive X-ray spectroscopy (EDX) was performed. As shown in Fig. 1(c) and (d), the elemental ratios of Fe/Co are about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the FCO-NCs and FCO-NSs, respectively, which are consistent with the element proportions in the raw materials. Therefore, the formulas of the FCO-NC and FCO-NS samples can be expressed as Fe1.2Co1.8O4 and FeCo2O4, respectively.
2 in the FCO-NCs and FCO-NSs, respectively, which are consistent with the element proportions in the raw materials. Therefore, the formulas of the FCO-NC and FCO-NS samples can be expressed as Fe1.2Co1.8O4 and FeCo2O4, respectively.
The morphologies of the FCO samples were investigated using scanning electron microscopy (SEM). Fig. 2(a) shows the SEM image of the FCO-NP precursors, which have aggregated into bulks with irregular shapes. From Fig. 2(b), it can be observed that after calcination, small FCO-NPs still aggregated and formed large bulks. Fig. 2(c) and (d) show the morphologies of the FCO-NC precursors and the final product, respectively. The FCO-NC precursor exhibited a truncated cubic shape, and after heating in air, the FCO-NC samples also possessed a similar shape but with rough surfaces. Fig. 2(e) shows the SEM image of the FCO-NS precursors, which present a spherical morphology. As shown in Fig. 2(f), the FCO-NS final product also exhibits a nano-sized spherical shape, but with an average diameter smaller than that of the precursor. This phenomenon might be attributed to inward shrinkage during calcination. From the inner image in Fig. 2(f), it could be observed that the surfaces of the products are also very rough indicating that these samples are polycrystalline, made from smaller nanoparticles. To confirm the external structures of the FCO-NCs and FCO-NSs, these two samples were mechanically ground and the SEM images were taken again. As shown in Fig. S1,† nano-structures with broken shells and hollow spaces could be observed. FCO-NSs possessed much thicker walls than FCO-NCs and a small hollow center (shown by the white arrows), indicating the higher mechanical strength of the FCO-NSs than the FCO-NCs.
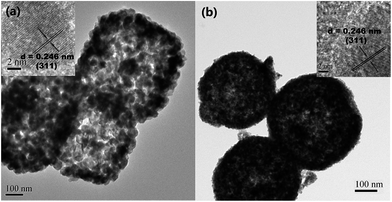
Transmission electron microscopy (TEM) was employed to further characterize the morphology and microstructure of the as-prepared FCO samples. As shown in Fig. 3(a) and (b), both the FCO-NCs and FCO-NSs have relatively compact shells and loose porous inner architectures. Nanocrystals were randomly selected, and their measured neighboring interlayer distances (0.246 nm) were in good agreement with the spacing between the (311) planes of the FeCo2O4 spinel phase,32 in good agreement with the XRD analysis.
To investigate the Brunauer–Emmett–Teller (BET) surface area and pore-size distribution of the FCO-NCs and FCO-NSs, the N2 sorption isotherms were collected and the results are shown in Fig. 4. The surface area of the FCO-NC sample was about 58.2 m2 g−1. This sample also possessed a porous feature with a pore size distribution (inset in Fig. 4) centered at around 11.6 nm, which could be derived from the controlled gas release during the calcination process. For the FCO-NS sample, the surface area was about 30.2 m2 g−1, which is smaller than that of the FCO-NCs, and its pore size distribution was wider than that of the FCO-NCs, centered at around 20.6 nm. The porous structure may allow for fast penetration of electrolyte through the pores and ample contact with the surfaces of primary particles, which might contribute to the considerable improvement of the electrochemical performance.36
According to the analysis of the above results, a schematic illustration of the preparation process of FCO-NCs and FCO-NSs is presented in Fig. 5. The morphologies of the FCO samples were controlled by the formation of their precursors. In the synthesis of the FCO-NCs, SDS acted as a surfactant to prevent the precursor nanoparticles from aggregating, so dispersed precursors were easily formed. During the growth of Co3[Fe(CN)6]2, facets with a lower surface energy were preferentially formed, and a truncated cubic shape was obtained. When the precursors were calcinated in air, the elements C and N were oxidized into gases and escaped, leaving pores in the secondary nano-structures. The formation of a hollow-core may be attributed to the Kirkendall effect, which has been widely used in the synthesis of porous materials. In the synthesis procedure of the FCO-NSs, SDBS was also a surfactant that formed micelles, yet it reacted with Co2+ to form Co(DBS)2 micelles. Co(DBS)2 could be quickly transformed to Co3[Fe(CN)6]2. When it reacted with [Fe(CN)6]3−, the morphology of the nanospheres was retained. No cubic precursor was formed during the growing stage. A possible reason may be related to the stronger alkalinity of SDBS. Alkaline could partially etch Co3[Fe(CN)6]2, especially on the edges with a higher surface energy, so the spherical shape remained. As a control, when no surfactant was used, the shape of Co3[Fe(CN)6]2 was irregular, and small particles were further aggregated into micro-sized bulks, which indicates that surfactants play a critical role on the final morphology of FCO.
A hollow porous architecture and high surface area are beneficial properties for anode materials of Li-ion batteries, so FeCo2O4 porous nanospheres may display an excellent electrochemical performance. To evaluate whether FCO samples with different morphologies were applicable in Li-ion batteries, the as-prepared products were used as anode materials in standard FCO/Li half-batteries. Fig. 6(a)–(c) show the first, second and 50th discharge curves of the FCO-NC, FCO-NS and FCO-NP electrodes at a current density of 100 mA g−1. The initial discharge shows a clear potential plateau at about 0.88 V vs. Li+/Li, at which the reduction of FCO to Fe and Co metal could happen. The following incline region may be related to the decomposition of the electrolyte catalyzed by metal nanoparticles. This decomposition reaction may cause the formation of a gel-like thin film of electrolyte on the surface of the materials, which can provide capacities besides oxidation and reduction. This electrolyte film can also prevent nanoparticles from aggregating during cycling, which reduces the decay of the capacity. After the first discharge, the flat plateau is substituted by a sloping curve. This may be because of a heterogeneous reaction mechanism of lithium insertion and extraction. The first discharge capacities of the FCO-NPs, FCO-NCs and FCO-NSs are 980, 1419 and 1359 mA h g−1, respectively, all of which are larger than the theoretical value. This is always ascribed to the irreversible reaction to form the SEI film. Gel-like electrolyte film may provide additional capacity45 and interfacial lithium storage may be another reason.46–48 The first charging capacities of the FCO-NPs, FCO-NCs and FCO-NSs are 657, 1116 and 1074 mA h g−1, respectively. Herein, the initial coulombic efficiencies can be calculated as about 67.0%, 78.6% and 79.1%, respectively. Fig. 6(d) shows the charge–discharge capacities versus cycle number for electrodes made from the FCO-NPs, FCO-NCs and FCO-NSs. Owing to the special morphology and structure, FCO-NS samples exhibit an excellent cycle performance. The capacity versus cycle number curves at a current density of 100 mA g−1 scanned between 3 and 0.01 V are shown. After 50 cycles, the discharge capacity of the FCO-NSs can still be retained at 1060 mA h g−1. However, the capacities of the FCO-NPs and FCO-NCs reach only 389 and 721 mA h g−1 after 50 cycles, which are much lower than that of the FCO-NSs.
To better understand the electrochemical behavior of the hollow porous FCO-NSs, we also investigated the rate performance with respect to Li+ insertion/extraction. To evaluate the rate capability, the FCO electrodes were cycled at various current densities (100–1000 mA g−1) and the charge–discharge curves are shown in Fig. 6(d). The FCO-NSs show a much better rate capability than the FCO-NPs and FCO-NCs. The discharge capacities at current densities of 100, 200, 500, and 1000 mA g−1 are 1289, 1190, 1027, and 823 mA h g−1, respectively. Upon altering the current density back to 100 mA g−1, a discharge capacity as high as 1038 mA h g−1 could be recovered. The better rate capability can be largely attributed to the pore structure of the FCO-NSs. The pore structure could shorten the transport length of Li+ and facilitate Li+ migration, and the internal hollow structure may also provide a “buffer zone” to alleviate the strain resulting from Li+ insertion and extraction. The reasons for the better rate capability of the FCO-NSs than the FCO-NCs may be largely attributed to the wider pore size distribution and higher Co content. The performance of the FCO-NSs demonstrates that this material has great potential as a high performance anode for lithium-ion batteries.
Since the rate capability is directly related to the impedance of the cells, the impedance tests were performed at the end of the rate performance tests and the spectra are shown in Fig. 6(f). The plots consist of a depressed semicircle in the high- and middle-frequency regions and a straight line in the low-frequency region. It is clear that the diameter of the semicircle of the FCO-NSs is the smallest indicating a low charge-transfer resistance (Rct). The reason for the lower Rct value of the FCO-NSs and FCO-NCs than that of the FCO-NPs may be attributed to the porous structure, which facilitates the Li+ and electron migration. The reasons for the lower Rct value of the FCO-NSs than the FCO-NCs may be largely attributed to the wider pore distribution and higher Co content, which facilitates the electron migration and increases the conductivity.
4. Conclusions
FCO hollow porous spheres and cubes have been synthesized from PBA in the presence of different surfactants through a simple co-precipitation procedure accompanied by calcination in air. In comparison with FCO-NCs, FCO-NSs show a much better electrochemical performance as an anode for lithium ion batteries. This can be largely attributed to the wider pore distribution, higher Co content and higher mechanical strength, which facilitate the Li+ and electron migration and subsequently improve the rate capability and cycling performance. At a current density of 100 mA h g−1, the discharge capacity of the FCO-NSs remains at 1060 mA h g−1 after 50 cycles, while a capacity of 823 mA h g−1 was obtained at a current density of 1000 mA h g−1. FCO-NSs shows great potential as an anode material for lithium-ion batteries.Acknowledgements
This work was supported by the State Key Project of Fundamental Research (Grants 2014CB931900 and 2012CB932504), “Hundred Talents Project” and Scientific Research Equipment Project of the Chinese Academy of Sciences.References
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS PubMed.
- Y. Wang, L. Gu, Y. Guo, H. Li, X. He, S. Tsukimoto, Y. Ikuhara and L. Wan, J. Am. Chem. Soc., 2012, 134, 7874–7879 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2014, 113, 5364–5457 CrossRef PubMed.
- Z. Ma, T. Li, Y. Huang, J. Liu, Y. Zhou and D. Xue, RSC Adv., 2013, 3, 7398–7402 RSC.
- K. Zhang, X. Han, Z. Hu, X. Zhang, Z. Tao and J. Chen, Chem. Soc. Rev., 2015, 44, 699–728 RSC.
- W. Jiang, W. Zeng, Z. Ma, Y. Pan, J. Lin and C. Lu, RSC Adv., 2014, 4, 41281–41286 RSC.
- W. Li, L. Xu and J. Chen, Adv. Funct. Mater., 2005, 15, 851–857 CrossRef CAS PubMed.
- Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265–270 CrossRef CAS PubMed.
- Z. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS PubMed.
- X. W. Lou, D. Deng, J. Y. Lee, J. Feng and L. A. Archer, Adv. Mater., 2008, 20, 258–262 CrossRef CAS PubMed.
- Z. Zhang, H. Chen, H. Che, Y. Wang and F. Su, Mater. Chem. Phys., 2013, 138, 593–600 CrossRef CAS PubMed.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef CAS PubMed.
- F. M. Courtel, H. Duncan, Y. Abu-Lebdeh and I. J. Davidson, J. Mater. Chem., 2011, 21, 10206–10218 RSC.
- H. Zhao, Z. Zheng, K. W. Wong, S. Wang, B. Huang and D. Li, Electrochem. Commun., 2007, 9, 2606–2610 CrossRef CAS PubMed.
- G. Zhang and X. W. David Lou, Sci. Rep., 2013, 3, 1470 Search PubMed.
- P. R. Kumar and S. Mitra, RSC Adv., 2013, 3, 25058–25064 RSC.
- C. Vidal-Abarca, P. Lavela and J. L. Tirado, J. Phys. Chem. C, 2010, 114, 12828–12832 CAS.
- X. Yao, J. Kong, X. Tang, D. Zhou, C. Zhao, R. Zhou and X. Lu, RSC Adv., 2014, 4, 27488–27492 RSC.
- Y. Sharma, N. Sharma, G. V. S. Rao and B. V. R. Chowdari, J. Power Sources, 2007, 173, 495–501 CrossRef CAS PubMed.
- Y. Sharma, N. Sharma, G. V. S. Rao and B. V. R. Chowdari, Solid State Ionics, 2008, 179, 587–597 CrossRef CAS PubMed.
- H. Kim, D. Seo, H. Kim, I. Park, J. Hong, K. Park and K. Kang, Chem. Mater., 2012, 24, 720–725 CrossRef CAS.
- Y. Deng, Q. Zhang, S. Tang, L. Zhang, S. Deng, Z. Shi and G. Chen, Chem. Commun., 2011, 47, 6828–6830 RSC.
- W. Kang, Y. Tang, W. Li, X. Yang, H. Xue, Q. Yang and C. S. Lee, Nanoscale, 2015, 7, 225–231 RSC.
- Y. Xiao, J. Zai, L. Tao, B. Li, Q. Han, C. Yu and X. Qian, Phys. Chem. Chem. Phys., 2013, 15, 3939–3945 RSC.
- L. Jin, Y. Qiu, H. Deng, W. Li, H. Li and S. Yang, Electrochim. Acta, 2011, 56, 9127–9132 CrossRef CAS PubMed.
- S. Liu, S. Zhang, Y. Xing, S. Wang, R. Lin, X. Wei and L. He, Electrochim. Acta, 2014, 150, 75–82 CrossRef CAS PubMed.
- B. Liu, J. Zhang, X. Wang, G. Chen, D. Chen, C. Zhou and G. Shen, Nano Lett., 2012, 12, 3005–3011 CrossRef CAS PubMed.
- P. F. Teh, Y. Sharma, S. S. Pramana and M. Srinivasan, J. Mater. Chem., 2011, 21, 14999–15008 RSC.
- C. T. Cherian, J. Sundaramurthy, M. V. Reddy, P. Suresh Kumar, K. Mani, D. Pliszka, C. H. Sow, S. Ramakrishna and B. V. R. Chowdari, ACS Appl. Mater. Interfaces, 2013, 5, 9957–9963 CAS.
- G. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed.
- Z. Li, T. Zhao, X. Zhan, D. Gao, Q. Xiao and G. Lei, Electrochim. Acta, 2010, 55, 4594–4598 CrossRef CAS PubMed.
- L. Zhou, D. Zhao and X. W. Lou, Adv. Mater., 2012, 24, 745–748 CrossRef CAS PubMed.
- J. Li, S. Xiong, Y. Liu, Z. Ju and Y. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 CAS.
- Z. Zhang, Y. Wang, Q. Tan, Z. Zhong and F. Su, J. Colloid Interface Sci., 2013, 398, 185–192 CrossRef CAS PubMed.
- J. Li, J. Wang, X. Liang, Z. Zhang, H. Liu, Y. Qian and S. Xiong, ACS Appl. Mater. Interfaces, 2014, 6, 24–30 CAS.
- Z. Zhang, Q. Tan, Y. Chen, J. Yang and F. Su, J. Mater. Chem. A, 2014, 2, 5041–5050 CAS.
- S. G. Mohamed, C. J. Chen, C. K. Chen, S. F. Hu and R. S. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 22701–22708 CAS.
- A. K. Rai, J. Gim, T. V. Thi, D. Ahn, S. J. Cho and J. Kim, J. Phys. Chem. C, 2014, 118, 11234–11243 CAS.
- T. Rodenas, I. Luz, G. Prieto, B. Seoane, H. Miro, A. Corma, F. Kapteijn, F. X. Llabrés i Xamena and J. Gascon, Nat. Mater., 2015, 14, 48–55 CrossRef CAS PubMed.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gándara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O’Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- R. Wu, X. Qian, F. Yu, H. Liu, K. Zhou, J. Wei and Y. Huang, J. Mater. Chem. A, 2013, 1, 11126–11129 CAS.
- R. Wu, X. Qian, K. Zhou, J. Wei, J. Lou and P. M. Ajayan, ACS Nano, 2014, 8, 6297–6303 CrossRef CAS PubMed.
- L. Zhang, H. B. Wu, S. Madhavi, H. H. Hng and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 17388–17391 CrossRef CAS PubMed.
- Z. Li, B. Li, L. Yin and Y. Qi, ACS Appl. Mater. Interfaces, 2014, 6, 8098–8107 CAS.
- J. Jamnik and J. Maier, Phys. Chem. Chem. Phys., 2003, 5, 5215–5220 RSC.
- H. Li, P. Balaya and J. Maier, J. Electrochem. Soc., 2004, 151, A1878–A1885 CrossRef CAS PubMed.
- P. Balaya, H. Li, L. Kienle and J. Maier, Adv. Funct. Mater., 2003, 13, 621–625 CrossRef CAS PubMed.
- N. Yan, L. Hu, Y. Li, Y. Wang, H. Zhong, X. Hu, X. Kong and Q. Chen, J. Phys. Chem. C, 2012, 116, 7227–7235 CAS.
- L. Hu, P. Zhang, H. Zhong, X. Zheng, N. Yan and Q. Chen, Chem.–Eur. J., 2012, 18, 15049–15056 CrossRef CAS PubMed.
- Z. Li, B. Li, L. Yin and Y. Qi, ACS Appl. Mater. Interfaces, 2014, 6, 8098–8107 CAS.
- F. Zheng, D. Zhu, X. Shi and Q. Chen, J. Mater. Chem. A, 2015, 3, 2815–2824 CAS.
- G. Huang, L. Zhang, F. Zhang and L. Wang, Nanoscale, 2014, 6, 5509–5515 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02781k |
| This journal is © The Royal Society of Chemistry 2015 |