Deciphering the formation mechanism of a protective corrosion product layer from electrochemical and natural corrosion behaviors of a nanocrystalline zinc coating†
Abstract
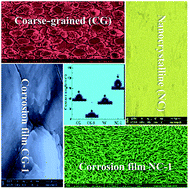
The corrosion resistance improvement of a zinc coating with the reduction of grain size from micro to nano-scale has long been attributed to the formation of better protection of the corrosion product layer. However, the formation mechanism of the protective corrosion product layer has rarely been studied. Here nanocrystalline zinc coatings are produced by pulse reverse electrodeposition in a sulfate bath with polyacrylamide as the only additive. The electrochemical and natural corrosion behaviors of an electrodeposited nanocrystalline zinc coating in comparison with a conventional coarse-grained zinc coating in simulated seawater are investigated. The nanocrystalline zinc coating exhibits distinctly enhanced corrosion resistance in the simulated seawater compared to the coarse-grained zinc coating. The enhanced corrosion resistance of zinc coatings with the reduction of grain size from micro (6 μm) to nano-scale (31 nm) is due to the fact that the nanocrystalline zinc coating is characterized by a high-volume fraction of grain boundaries, and the zinc atoms at grain boundaries possess a higher activity. This is beneficial for rapidly forming a protective corrosion product film with a hydrophobic nano-wire structure on the surface of the zinc coating during the exposure to simulated seawater, thereby contributing to the corrosion resistance enhancement. Based on analysis results, the possible formation mechanism of a protective corrosion product layer on the surface of the nanocrystalline zinc coating is discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...