Cu(I) complex based on 6H-indolo[2,3-b]quinoxaline: structure and electrocatalytic properties for hydrogen evolution reaction from water†
Pan Zhanga,
Xin Yangb,
Penggang Jianga,
Junli Yina,
Yun Gong*a and
Jianhua Lin*ac
aDepartment of Applied Chemistry, College of Chemistry and Chemical Engineering, Chongqing University, Chongqing 400030, P. R. China. E-mail: gongyun7211@cqu.edu.cn; Tel: +86-023-65106150
bChongqing Foreign Language School, Chongqing 400039, P. R. China
cState Key Laboratory of Rare Earth Materials Chemistry and Applications, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, P. R. China. E-mail: jhlin@pku.edu.cn; jhlin@cqu.edu.cn; Tel: +86-010-62753541
First published on 7th April 2015
Abstract
Using a rigid ligand, 6H-indolo[2,3-b]quinoxaline (HL), a Cu(I) complex formulated as Cu2L2 (1) has been solvothermally synthesized and structurally characterized by single-crystal X-ray diffraction. The complex is a planar Cu2 dimer, in which two Cu(I) ions are linked by two L− with a Cu⋯Cu separation of 2.637 Å. And different Cu2 units are connected by strong π–π stacking interactions into a three-dimensional (3D) supramolecular architecture. The HL ligand can't electrocatalyze the hydrogen evolution reaction (HER) from water, whereas the Cu(I) complex can act as an electrochemically stable electrocatalyst. In the presence of the Cu(I) complex, the exchanging current density i0 for the HER is improved. And with the increase of the temperature, the HER current density is increased, and the average activation energy Ea from 30 to 50 °C for the HER in the presence of the complex is calculated as ca. 40 kJ mol−1.
Introduction
In recent years, metal complexes have attracted people's great interest due to their intriguing variety of structures and interesting properties in catalysis, porous materials, etc.1 In particular, metal complexes have received much attention in electrochemical applications.2 For example, Mao et al. used copper-based metal complexes as electrocatalysts in the oxygen reduction reactions (ORR).2a Kumar et al. reported the electrocatalytic reduction of carbon dioxide at copper-based metal complex surface.2b In this regard, our research has been mainly focused on the synthesis and characterization of novel metal complexes to investigate their electrocatalytic activities for the H2 evolution reaction (HER) from water.3In our previous work, it is found that the species of metal(II) ions plays an important role in the electrocatalytic activity for the HER.3 Usually the Co(II), Ni(II) or Cu(II) complex possesses the electrocatalytic activity for the HER, whereas the Zn(II) or Cd(II) complex doesn't have. And the sequence for the electrocatalytic activity is: Co(II) complex > Ni(II) complex > Cu(II) complex.3 Metal complex is usually not electroconductive, which limits its uses in electrochemical field. To solve the problem, our strategy is to synthesize its graphene composite material to improve its electroconductivity.3a Lee et al. reported a composite material of graphene oxide and copper-centred metal complex, which shows good performances as a tri-functional electrocatalyst in the HER, oxygen evolution reaction (OER) and ORR.2c
However, the kinetic factor such as the activation energy Ea in the catalysis for the HER is still unexplored, herein, in order to investigate the relationship between the temperature and the electrocatalytic activity of metal complex for the HER, we synthesized a rigid ligand, 6H-indolo[2,3-b]quinoxaline (HL) (Scheme 1).4 It is expected the ligand is stable under the electrochemical condition. Based on HL, we got a Cu(I) complex formulated as Cu2L2 (1). The cyclic voltammograms (CVs), Tafel plots, electrochemical impedance spectroscopies (EISs), controlled potential electrolysis (CPE) experiments under the temperature of 30, 50 and 80 °C as well as the UV-vis absorption spectra, electrochemical stability and thermal stability of the complex have been investigated.
Experimental section
General considerations
All chemicals purchased were of reagent grade and used without further purification. The melting point was determined using an uncorrected X-4 melting point apparatus of Beijing Kaifu Company. C, H, N elemental analyses were performed on an Elementar Vario MICRO E III analyzer. IR spectra were recorded as KBr pellets on PerkinElmer spectrometer. The powder X-ray diffraction (PXRD) data were collected on a RIGAKU DMAX2500PC diffractometer using Cu Kα radiation. Thermogravimetric analysis (TGA) and simultaneous differential thermal analysis (DTA) were performed on a NETZSCH STA 449C thermogravimetric analyzer in flowing N2 with a heating rate of 10 °C min−1. The morphologies of the samples were characterized by SEM (Model JSM-7600F, JEOL). UV-vis spectra were measured on a HITACHI U-4100 UV-vis spectro-photometer.Electrochemical measurements
The electrochemical measurements were done in a three-electrode test cell using a Shiruisi RST5200 electrochemical workstation. A saturated calomel electrode (SCE) and a platinum foil were used as the reference and counter electrode, respectively. The working electrode was prepared as follows: firstly, a glassy carbon electrode (GCE) was polished by abrasive paper and aluminum oxide, then ultrasonically washed by ethanol, acetone and distilled water. Then an acetone dispersion of 4 mg HL or complex 1 and 0.05 mL of Nafion were deposited on the GCE and the solvent is dried by an IR lamp. The electroactive geometric area of the GCE is 0.2 cm2. The measurements were recorded in 50 mL of N2 degassed 0.05 M phosphate buffer (pH = 6.8, 50 mL) aqueous solution. The amount of H2 evolved was determined using gas chromatography (GC, 7890A, thermal conductivity detector (TCD), Ar carrier, Agilent). Electrochemical impedance spectroscopy (EIS) measurements were conducted on a CHI660E electrochemical workstation in the range of 0.01 Hz–1 MHz, and the experimental conditions of EIS are as follows: the amplitude of the potential perturbation is 0.005 V.X-ray crystallography
Single-crystal X-ray data for HL and complex 1 were collected on an Oxford XCalibur Eos diffractometer using graphite monochromated Mo Kα (λ = 0.71073 Å) radiation at room temperature. Empirical absorption correction was applied. The structures were solved by direct methods and refined by the full-matrix least-squares methods on F2 using the SHELXTL-97 software.5 All non-hydrogen atoms were refined anisotropically. All of the hydrogen atoms were placed in the calculated positions. The crystal data and structure refinements for HL and complex 1 are summarized in Table 1. Selected bond lengths and angles for HL and complex 1 are listed in Table S1 in the ESI.†| a R1 = Σ‖F0| − |Fc‖/Σ|F0|; wR2 = Σ[w(F02 − FC2)2]/Σ[w(F02)2]1/2. | ||
|---|---|---|
| Complex | HL | 1 |
| Empirical formula | C14H9N3 | Cu2C28H16N6 |
| M | 219.24 | 563.55 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/n |
| a/Å | 5.9177(5) | 8.3236(3) |
| b/Å | 9.092(8) | 5.6787(2) |
| c/Å | 18.7984(19) | 22.6705(7) |
| α/° | 90 | 90 |
| β/° | 91.9470(10) | 90.581(3) |
| γ/° | 90 | 90 |
| V/Å3 | 1010.9(9) | 1071.52(6) |
| Z | 4 | 2 |
| Dcalcd/g cm−3 | 1.441 | 1.747 |
| μ/mm−1 | 0.089 | 2.018 |
| No. of unique reflections | 1770 | 1887 |
| Reflections used [I > 2σ(I)] | 974 | 1238 |
| F(0 0 0) | 456 | 568 |
| Goodness-of-fit on F2 | 1.048 | 1.100 |
| Final R indices [I > 2σ(I)] | R1 = 0.0754, wR2 = 0.1659 | R1 = 0.0472, wR2 = 0.1207 |
Results and discussion
Crystal structure of HL and Cu2L2 (1)
Single-crystal X-ray diffraction analysis reveals that HL and complex 1 both crystallize in the monoclinic space group P21/n. In HL, there is one crystallographically independent HL molecule in the asymmetric unit. HL is a planar molecule with 1.346(5) and 1.392(5) Å of the two C–N distances in the pyrrole ring, and the two neighboring C–N bond lengths in the pyrazine ring are 1.314(5) and 1.376(5) Å, respectively (Table S2†).In complex 1, the asymmetric unit contains one Cu(I) and one L−. As shown in Fig. 1a, in complex 1, the crystallographically independent Cu(1) exhibits a linear geometry, defined by one pyrrole N atom and one pyrazine N atom from two L− [Cu–N 1.863(4)–1.889(4) Å] (Fig. 1a). Valences sum calculation shows that the Cu atom is in the +1 oxidation state6 though the complex was synthesize based on Cu(II) salt. The work indicates that the Cu(II) ion in the starting material has been reduced to Cu(I) in the synthesis of the complex, which is because Cu(I) possesses more stable electron configuration (3d10) than Cu(II).
In the complex, the L− is planar with the H atom attached to the pyrrole N atom deprotonated. The C–N bond lengths in the pyrrole ring and pyrazine ring in L− are 1.338(6), 1.405(6), 1.323(6) and 1.389(5) Å, respectively (Table S2†), indicating the C–N distances in the L− and HL ligands are a bit different, which is probably due to the difference of electron densities before and after the deprotonation. Two Cu(I) ions are linked by two L− molecules via two pyrrole N atoms and two pyrazine N atoms into a Cu2 unit with a Cu⋯Cu separation of 2.637(11) Å (Fig. 1a). And all the atoms in the Cu2 unit are coplanar (Fig. 1a). Different units are connected by strong π–π stacking interactions (Table S2†) into a three-dimensional (3D) supramolecular architecture (Fig. 1b).
Thermal stability of complex 1
The powder X-ray diffraction (PXRD) of complex 1 is shown in Fig. S1 in the ESI.† All the peaks of the compound can be indexed to the simulated XRD powder pattern, indicating the compound is pure phase. In order to examine the thermal stability of complex 1, thermogravimetric analysis (TGA) and simultaneous differential thermal analysis (DTA) were carried out. The sample was heated up to 750 °C in N2.As shown in Fig. 2, no weight loss is observed from room temperature to 430 °C, which is in good agreement with the crystal structure of complex 1, in which no solvent is included. The organic ligand began to be decomposed when the temperature is higher than 430 °C, and the calorimetric curve of complex 1 shows an obvious endotherm with a peak at 504 °C. The TGA curve of complex 1 shows that the compound possesses good thermal stability.
The electrochemical property of HL and complex 1
The electrochemical properties of HL and complex 1 were evaluated by cyclic voltammograms (CVs) in 0.05 M phosphate buffer aqueous solution (pH = 6.8, H3PO4/KOH, 50 mL) in a three-electrode cell with a SCE and a platinum foil were used as the reference and counter electrode, respectively. The CVs for the bare GCE, HL-modified glassy carbon electrode (L-GCE) and 1-modified electrode (1-GCE) were measured. As shown in Fig. 3 and S2,† under the room temperature of 30 °C, the bare GCE exhibits the proton reduction at an onset potential of approximately −1.84 V vs. SCE at a scan rate of 0.01 V s−1. Since in a neutral aqueous solution (pH = 6.8), the theoretical potential for the H2 evolution reaction (HER) on a clean Pt electrode is −0.64 V vs. SCE,7 the HER overpotential (η) at the bare GCE is 1.20 V vs. SCE. The weak reduction peak at ca. −0.96 V vs. SCE is probably associated with the impurity in the blank system.3 | ||
| Fig. 3 CVs of the bare GCE, HL-GCE and 1-GCE in 0.05 M phosphate buffer aqueous solution (pH = 6.8, H3PO4/KOH, 50 mL) at a sweep rate of 0.01 V s−1 at 30 °C. | ||
As shown in Fig. 3 and S3,† under the same temperature (30 °C) and at the same scan rate, the CVs reveal the proton reduction peak at HL-GCE was negatively shifted with respect to the bare GCE, indicating the free ligand HL can't catalyze the HER. And the irreversible reduction peak of the impurity in the blank system (−0.96 V vs. SCE), is shifted to −0.86 V vs. SCE in the presence of HL (Fig. 3).
However, under the room temperature (30 °C), 1-GCE shows the lower HER potential (−1.64 V vs. SCE, η = 1.00 V) at 0.01 V s−1 and the HER current density is enhanced with respect to the bare GCE (Fig. 3 and S4†), inferring that complex 1 can act as an electrocatalyst for the generation of H2.8 The quasi-reversible peaks at −0.12 and + 0.32 V vs. SCE may be ascribed to the redox of Cu(I)/Cu(II) in complex 1 (Fig. 3 and S4†).3c,9 Similarly, the weak reduction peak at −0.70 V vs. SCE is also probably associated with the redox of the impurity in the system.3
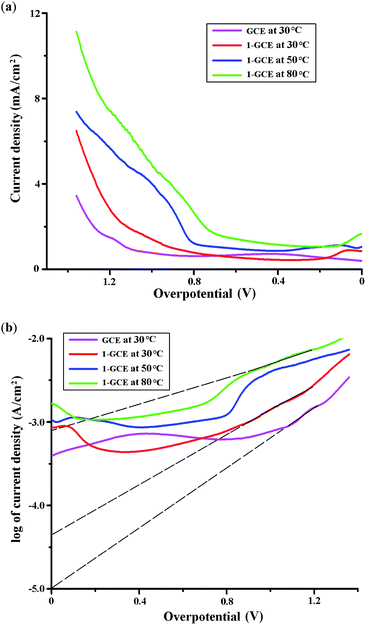
The electrocatalytic activity of complex 1 for the HER at 30 °C was also proved by the current intensity/overpotential diagram and the Tafel plot. As shown in Fig. 4a and 4b, when the scan rate is 0.01 V s−1, 1-GCE shows lower overpotential and enhanced current density for the HER with respect to the bare GCE. According to η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i, the intercept at the y axis a = log
i, the intercept at the y axis a = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 (i0 is the exchanging current density), the slope b = 2.303RT/(αnF) (α is the charge-transfer efficiency).10 As shown in Fig. 4b, in the presence of complex 1, a = −4.4 and b = −0.66, it can be calculated α = 4.6 × 10−2 and i0 = 4.0 × 10−5 A. Whereas without the electrocatalyst of complex 1, a = −5.0 and b = −0.55, then α = 5.5 × 10−2 and i0 = 1.0 × 10−5 A. The result indicates that the presence of complex 1 can improve the exchanging current density i0 with respect to the bare GCE.
i0 (i0 is the exchanging current density), the slope b = 2.303RT/(αnF) (α is the charge-transfer efficiency).10 As shown in Fig. 4b, in the presence of complex 1, a = −4.4 and b = −0.66, it can be calculated α = 4.6 × 10−2 and i0 = 4.0 × 10−5 A. Whereas without the electrocatalyst of complex 1, a = −5.0 and b = −0.55, then α = 5.5 × 10−2 and i0 = 1.0 × 10−5 A. The result indicates that the presence of complex 1 can improve the exchanging current density i0 with respect to the bare GCE.
When the scan rate was changed to be 0.005 V s−1, similar phenomenon is also observed in the Tafel plot, as shown in Fig. S5a,† in which the intercept a = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i0 for 1-GCE is also larger than the bare GCE, indicating the exchanging current density i0 for the HER is improved at 1-GCE.
i0 for 1-GCE is also larger than the bare GCE, indicating the exchanging current density i0 for the HER is improved at 1-GCE.
At an electrolysis potential of −1.64 V vs. SCE (η = 1.00 V), in the presence of complex 1, with the increase of scan rate v, the ratio of the HER peak current ip/square root of the scan rate v1/2 is decreased (Fig. S6†), indicating the rate determining step of the hydrogen evolution is electrochemical reaction.10a
The electrochemical impedance spectroscopy (EIS) of the complex was measured under the temperature of 30 °C. The Bode plots of 1-GCE and the bare GCE at −1.5 V vs. SCE are shown in Fig. S7.† The Rs (electrolyte resistance) and the sum of Rs and Rct (charge-transfer resistance) can be observed from the magnitude plot in the high and low frequency regions, respectively.11 It is found that both the Rs and the sum of Rs and Rct for the HER in the presence of complex 1 is lower than that in the blank system, indicating the resistance of the charge transfer is lowered in the presence of the complex.11 It is probably associated with the Cu(I) ion in the complex, which function as the active center for the binding of intermediate species.12
Controlled potential electrolysis (CPE) experiment over 1 h at −1.5 V vs. SCE (η = 0.86 V) under the temperature of 30 °C was performed to investigate the electrocatalytic property of the complex for the HER. As depicted in Fig. S8a and S8b,† 1-GCE shows more charge buildup versus time than the bare GCE, and larger HER current density is observed at 1-GCE than the bare GCE under similar condition. And CPE experiment reveals the electrocatalyst of complex 1 operates at 98% Faradaic efficiency for the HER (see ESI†) with a turnover number (TON) of 1.2 mol of H2 per mole of complex 1 at 30 °C.
Moreover, 1-GCE is stable. When the potential range is maintained at −2.0 to 2.0 V vs. SCE, the peak current can be kept one hundred of cycles. After the CV cycles, the solid sample left on the 1-GCE (Fig. S9†) is characterized by the powder X-ray diffraction (PXRD) (Fig. S1†), which displays a PXRD pattern similar to that of complex 1, indicating the sample left on the electrode retain its structural integrity under the HER condition. The result is also proved by the SEM images of the sample before and after the electrochemical measurements. As shown in Fig. 5 and S10,† it is found that the morphologies of the sample are similar before and after the CV cycles.
 | ||
| Fig. 5 The SEM images of complex 1 before (a) and after the electrochemical experiments at 30 °C (b), 50 °C (c) and 80 °C (d), respectively. | ||
The electrolyte solution before and after the CV cycles was characterized by UV-vis spectroscopy. As shown in Fig. S11,† the solution exhibits similar absorption spectra before and after the electrochemical experiment, indicating complex 1 wasn't decomposed during the electrocatalytic process for the HER.
The effect of temperature on the electrocatalytic property of complex 1
In order to investigate the effect of temperature on the electrocatalytic property of complex 1 for the HER, the CVs for 1-GCE were also measured under similar condition at 50 and 80 °C, respectively.As shown in Fig. 6, S12 and S13,† the quasi-reversible couple of −0.12/+0.32 V vs. SCE at 1-GCE under the temperature of 30 °C was shifted to −0.12/+0.17 and −0.07/+0.04 V vs. SCE at 50 and 80 °C, respectively, which might be attributed to the redox of the Cu ion in complex 1.3c,9 And the irreversible reduction peak was appeared at −0.70, −0.66 and −0.63 V vs. SCE under the temperature of 30, 50 and 80 °C, respectively, which is probably ascribed to the redox of the impurity in the system.3 As shown in Fig. 6, it is found that the HER current density is increased with the increase of the temperature. For example, when the scan rate is 0.01 V s−1, at −1.64 V vs. SCE (η = 1.00 V), as shown in Fig. 4a, the HER current density is 1.37 and 4.00 mA cm−2 at 30 and 50 °C, respectively. According to Arrhenius equation, k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/RT), ln(k2/k1) = Ea/R{(T2 − T1)/(T1T2)}, in which k1 and k2 are the rate constant at the temperature of T1 and T2, respectively. Herein, the rate constant is proportional to the HER current density. Then the average activation energy Ea in the temperature range from 30 to 50 °C is calculated to be 43.6 kJ mol−1. Similarly, when the scan rate is 0.005 V s−1, at the overpotential η of 1.00 V, as shown in Fig. 5b, the HER current density is 1.25 and 3.40 mA cm−2 at 30 and 50 °C, respectively, and the Ea in the temperature range of 30–50 °C is calculated as 40.6 kJ mol−1.
exp(−Ea/RT), ln(k2/k1) = Ea/R{(T2 − T1)/(T1T2)}, in which k1 and k2 are the rate constant at the temperature of T1 and T2, respectively. Herein, the rate constant is proportional to the HER current density. Then the average activation energy Ea in the temperature range from 30 to 50 °C is calculated to be 43.6 kJ mol−1. Similarly, when the scan rate is 0.005 V s−1, at the overpotential η of 1.00 V, as shown in Fig. 5b, the HER current density is 1.25 and 3.40 mA cm−2 at 30 and 50 °C, respectively, and the Ea in the temperature range of 30–50 °C is calculated as 40.6 kJ mol−1.
 | ||
| Fig. 6 CVs of 1-GCE in 0.05 M phosphate buffer aqueous solution (pH = 6.8, H3PO4/KOH, 50 mL) at a sweep rate of 0.01 V s−1 at 30 °C (red), 50 °C (blue) and 80 °C (green), respectively. | ||
The Tafel plots at different temperatures are shown in Fig. 4b. It is found that at 80 °C, the intercept a = −3.1 and the exchanging current density i0 = 7.9 × 10−4 A, which is higher than the values at 30 °C (a = −5.0, i0 = 5.0 × 10−5 A), indicating that with the increase of the temperature, the exchanging current density is increased.
The EISs of complex 1 at different temperatures are shown in Fig. S7.† It is observed that both the Rs and the sum of Rs and Rct for the HER at 1-GCE are lowered with the increase of the temperature, indicating the increase of the temperature can prompt the diffusion of electrolyte and the transfer of charge.
The results of 1h-CPE at −1.5 V vs. SCE (η = 0.86 V) under different temperatures are in Fig. S8.† 1-GCE shows more charge buildup versus time and larger HER current density with the increase of the temperature. And complex 1 operates at 99% Faradaic efficiencies for the HER (see ESI) with a TON of 2.7 and 4.6 mol of H2 per mole of complex 1 at 50 and 80 °C, respectively.
As shown in Fig. S1,† the solid sample left on the 1-GCE after one hundred of CV cycles in the potential range of −2.0 to 2.0 V vs. SCE at 50 and 80 °C also exhibits a PXRD pattern similar to that of complex 1. And the sample can keep its morphology after the CV cycles at different temperatures, as shown in Fig. 5 and S10,† indicating the good electrochemical stability of complex 1 under different temperatures, which is probably associated with the quasi-reversible redox property of Cu(I)/Cu(II) in the complex (Fig. 3 and 6).
UV-vis absorption spectra
The UV-vis absorption spectra of the free organic ligand HL and complex 1 at room temperature are shown in Fig. S14.† As shown in Fig. S14,† the free ligand HL exhibits strong absorption peaks at ca. 250, 320 and 410 nm in the range of 230–500 nm, which may be ascribed to the n–π* or π–π* transition.13 Complex 1 displays absorption peaks at ca. 280, 340 and 390 nm in the range of 230–500 nm. The absorption peaks for complex 1 is different from those of HL, indicating they may be ascribed to the intraligand transition (ILCT) or metal-to-ligand charge-transfer transition (MLCT).13 In the range of 500–850 nm, HL and complex 1 show absorption peaks at ca. 535 and 550 nm, respectively, they may be ascribed to the visible d–d transition (Fig. S14†).Conclusion
Based on HL, a Cu(I) complex formulated as Cu2L2 (1) has been synthesized and structurally characterized by single-crystal X-ray diffraction. The complex is a planar Cu2 dimer, in which two Cu(I) ions are linked by two L− via two pyrrole N atoms and two pyrazine N atoms with a Cu⋯Cu separation of 2.637 Å. And different Cu2 units are connected by strong π–π stacking interactions into a 3D supramolecular architecture.The HL ligand can't electrocatalyze the HER from water, whereas the Cu(I) complex can act as the electrocatalyst, which is probably because the Cu(I) ion in the complex functions as the active center for the binding of intermediate species during the HER.12 In the presence of the Cu(I) complex, both the Rs and the sum of Rs and Rct for the HER are lowered, and the exchanging current density i0 for the HER is improved. And with the increase of the temperature, the HER current density in the presence of the complex is increased, and it is calculated the average activation energy Ea in the temperature range from 30 to 50 °C for the HER is ca. 40 kJ mol−1. Before and after one hundred of CV cycles in the potential range of −2.0 to 2.0 V vs. SCE, the sample on the electrode shows similar PXRD pattern and similar morphology, indicating the good electrochemical stability of the complex, which is probably associated with the quasi-reversible redox property of Cu(I)/Cu(II) in the complex.
Financial supports from the National Natural Science Foundation of China (no. 21371184), the Fundamental Research Funds for the Central Universities (no. CQDXWL-2012-024), the large-scale instrument and equipment open foundation in Chongqing University (no. 201406150041) and Chongqing Key Laboratory of Chemical Process for Clean Energy and Resource Utilization are gratefully acknowledged.
References
-
(a) M. W. Hosseini, Acc. Chem. Res., 2005, 38, 313 CrossRef CAS PubMed
; (b) P. J. Steel, Acc. Chem. Res., 2005, 38, 243 CrossRef CAS PubMed
; (c) C. Janiak, Coord. Chem. Rev., 2006, 250, 66 CrossRef CAS PubMed
; (d) F. Blank and C. Janiak, Coord. Chem. Rev., 2009, 253, 827 CrossRef CAS PubMed
; (e) X. C. Huang, Y. Y. Lin, J. P. Zhang and X. M. Chen, Angew. Chem., Int. Ed., 2006, 45, 1557 CrossRef CAS PubMed
; (f) Q. R. Fang, G. S. Zhu, Z. Jin, M. Xue, X. Wei, D. J. Wang and S. L. Qiu, Angew. Chem., Int. Ed., 2006, 45, 6126 CrossRef CAS PubMed
; (g) S. T. Zheng, J. Zhang, X. X. Li, W. H. Fang and G. Y. Yang, J. Am. Chem. Soc., 2010, 132, 15102 CrossRef CAS PubMed
.
-
(a) J. Mao, L. Yang, P. Yu, X. Wei and L. Mao, Electrochem. Commun., 2012, 19, 29 CrossRef CAS PubMed
; (b) R. Senthil Kumar, S. Senthil Kumar and M. Anbu Kulandainathan, Electrochem. Commun., 2012, 25, 70 CrossRef CAS PubMed
; (c) M. Jahan, Z. Liu and K. P. Loh, Adv. Funct. Mater., 2013, 23, 5363 CrossRef CAS PubMed
; (d) Y. F. Zhang, X. J. Bo, A. Nsabimana, C. Han, M. Li and L. P. Guo, J. Mater. Chem. A, 2015, 3, 732 RSC
; (e) M. Jahan, Q. L. Bao and K. P. Loh, J. Am. Chem. Soc., 2012, 134, 6707 CrossRef CAS PubMed
.
-
(a) X. L. Gao, Y. Gong, P. Zhang, Y. X. Yang, J. P. Meng, M. M. Zhang, J. L. Yin and J. H. Lin, CrystEngComm, 2014, 16, 8492 RSC
; (b) Y. Gong, M. M. Zhang, P. Zhang, H. F. Shi, P. G. Jiang and J. H. Lin, CrystEngComm, 2014, 16, 9882 RSC
; (c) Y. Gong, H. F. Shi, Z. Hao, W. Hua and J. H. Lin, Cryst. Growth Des., 2014, 14, 649 CrossRef CAS
.
-
(a) S. Takekuma, S. Katayama and H. Takekuma, Chem. Lett., 2000, 614 CrossRef CAS
; (b) K. Niume, S. Kurosawa, F. Toda, M. Hasegawa and Y. Iwakura, Bull. Chem. Soc. Jpn., 1982, 55, 2293 CrossRef CAS
; (c) L. M. Wilhelmsson, N. Kingi and J. Bergman, J. Med. Chem., 2008, 51, 7744 CrossRef CAS PubMed
.
- G. M. Sheldrick, SHELXS 97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997 Search PubMed
.
- N. E. Brese and M. O'keeffe, Acta Crystallogr., Sect. B: Struct. Sci., 1991, 47, 192 CrossRef
.
- B. Nohra, H. E. Moll, L. M. R. Albelo, P. Mialane, J. Marrot, C. M. Draznieks, M. O'Keeffe, R. N. Biboum, J. Lemaire, B. Keita, L. Nadjo and A. Dolbecq, J. Am. Chem. Soc., 2011, 133, 13363 CrossRef CAS PubMed
.
-
(a) L. Cheng, X. M. Zhang, X. D. Xi and S. J. Dong, J. Electroanal. Chem., 1996, 407, 97 CrossRef
; (b) X. L. Wang, C. Qin, E. B. Wang, Z. M. Su, Y. G. Li and L. Xu, Angew. Chem., Int. Ed., 2006, 45, 7411 CrossRef CAS PubMed
; (c) P. P. Zhang, J. Peng, H. J. Pang, J. Q. Sha, M. Zhu, D. D. Wang and M. G. Liu, CrystEngComm, 2011, 13, 3832 RSC
; (d) L. F. Yang, S. Kinoshita, T. Yamada, S. Kanda, H. Kitagawa, M. Tokunaga, T. Ishimoto, T. Ogura, R. Nagumo, A. Miyamoto and M. Koyama, Angew. Chem., Int. Ed., 2010, 49, 5348 CrossRef CAS PubMed
; (e) X. L. Wang, H. L. Hu, G. C. Liu, H. Y. Lin and A. X. Tian, Chem. Commun., 2010, 46, 6485 RSC
.
- L. F. Yang, S. Kinoshita, T. Yamada, S. Kanda, H. Kitagawa, M. Tokunaga, T. Ishimoto, T. Ogura, R. Nagumo, A. Miyamoto and M. Koyama, Angew. Chem., Int. Ed., 2010, 49, 5348 CrossRef CAS PubMed
.
-
(a) H. Yang and W. Q. Lu, Applied electrochemistry, Science press, Beijing, 2001, p. 46 Search PubMed
; (b) Y. Gao and B. Wu, Electrochemical Basis, Chemical industry press, Beijing, 2003, p. 53 Search PubMed
.
-
(a) M. Hunsom, Spectrosc. Prop. Inorg. Organomet. Compd., 2012, 42, 196 CAS
; (b) S. A. Mamuru, K. I. Ozoemena, T. Fukuda and N. Kobayashi, J. Mater. Chem., 2010, 20, 10705 RSC
; (c) S. Ghosh, R. K. Sahu and C. R. Raj, J. Mater. Chem., 2011, 21, 11973 RSC
; (d) M. K. Datta, K. Kadakia, O. I. Velikokhatnyi, P. H. Jampani, S. J. Chung, J. A. Poston, A. Manivannan and P. N. Kumta, J. Mater. Chem. A, 2013, 1, 4026 RSC
; (e) R. N. Reddy and R. G. Reddy, J. Power Sources, 2004, 132, 315 CrossRef CAS PubMed
.
- M. T. M. Koper and E. Bouwman, Angew. Chem., Int. Ed., 2010, 49, 3723 CrossRef CAS PubMed
.
-
(a) S. Ohkoshi, H. Tokoro, T. Hozumi, Y. Zhang, K. Hashimoto, C. Mathonière, I. Bord, G. Rombaut, M. Verelst, C. C. Moulin and F. Villain, J. Am. Chem. Soc., 2006, 128, 270 CrossRef CAS PubMed
; (b) M. Stadler, F. Puntoriero, S. Campagna, N. Kyritsakas, R. Welter and J. M. Lehn, Chem.–Eur. J., 2005, 11, 3997 CrossRef PubMed
; (c) J. H. Wang, Y. Q. Fang, L. Bourget-Merle, M. I. J. Polson, G. S. Hanan, A. Juris, F. Loiseau and S. Campagna, Chem.–Eur. J., 2006, 12, 8539 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data; PXRD patterns; CVs; Tafel plots; Bode plots; CPE plots; SEM images; the images of electrode; UV-vis absorption spectra; the electrochemical property of nanosized Pt and other supplementary material. CCDC 1046463 and 800789. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra02769a |
| This journal is © The Royal Society of Chemistry 2015 |