Fluorescent phenylethynylene calix[4]arenes for sensing TNT in aqueous media and vapor phase†
Kanokthorn Boonkitpatarakula,
Yamonporn Yodtab,
Nakorn Niamnontc and
Mongkol Sukwattanasinitt*b
aProgram of Petrochemistry, Faculty of Science, Chulalongkorn University, Bangkok 10300, Thailand. E-mail: keng_knb@hotmail.com; Tel: +66 81 815 4973
bDepartment of Chemistry, Faculty of Science, Chulalongkorn University, Bangkok 10300, Thailand. E-mail: msukwatt@gmail.com; Tel: +66 81 901 0730
cDepartment of Chemistry, Faculty of Science, King Mongkut's University of Technology Thonburi, Bangkok 10140, Thailand
First published on 30th March 2015
Abstract
The upper-rim of calix[4]arene is modified with phenylacetylene derivatives via Sonogashira coupling reaction to produce a wider cavity for entrapping TNT. The modified calix[4]arene with amino groups on the wider rim exhibits highly selective fluorescence quenching toward TNT compared to other nitro aromatic compounds in an aqueous medium due to the shape and hydrophobicity of the modified cavity. The limit of detection of TNT is 0.3 μM (68 ppb) in water and the Stern–Volmer fluorescence quenching constant is 1.09 × 105 M−1.
Introduction
Trinitrotoluene (TNT) is one of the most commonly used landmine and military explosives, partly owing to its insensitivity to shock and friction.1 Besides being highly explosive, TNT has also been recognized as a toxic substance causing skin irritation, anemia, liver cancer and also adversely affecting male fertility.1 TNT can contaminate the environment through armament processing. TNT concentrations in ground water and soil in the vicinity of munitions plants can be easily over 500 ppb.1a According to the US Environmental Protection Agency (EPA), the limit of TNT in drinking water is 2 ppb.2 For these reasons, the development of sensors for quick and facile detection of TNT is of great importance in environmental monitoring and remedying, detecting buried unexploded ordnance, and locating underwater mines.3Various techniques for TNT detections are available such as chromatography, surface-enhanced Raman spectroscopy, amperometry, X-ray dispersion, cyclic voltammetry and ion mobility spectrometry.3a,4 Among various sensing techniques available, fluorescence chemosensors have attracted much attention due to their high sensitivity and selectivity, short response time, cost-effectiveness in instrumentation and also operational simplicity suitable for on-site analysis.5 In particular, fluorescent conjugated polymers including poly(phenylene ethynylene),6 polymetalloles,7 poly(p-phenylene vinylenes)8 and diaryl poly(acetylenes)8b,9 have been widely studied.10 These conjugated fluorescent polymers can give high fluorescence quenching sensitivity owing to excitonic migration amplifying mechanisms.11 Their solid state dense films however generally have low permeability for analyte molecules.12 Their random and unpredictable conformation of the polymer chains may also lead to molecular orientation at the film surface unsuitable for binding analysts. Recently, development of small molecules as fluorescent sensors has gain more attention due to their well-defined structures, monodispersity, simpler synthesis and purification allowing better understanding of structure–property relationships. However, single-molecule-based fluorescent sensors for explosive detection have been investigated at a much lesser extent and they are mostly applied in organic solvents.12,13 The fluorescent sensory materials for TNT detection based mainly on photoinduced electron transfer (PET) fluorescent quenching process. However, most of fluorescent compounds have low selectivity between nitrotoluene and nitrophenol derivatives because both classes are strong electron acceptors14 and trinitrophenol (TNP) usually appeared to give higher quenching efficiencies than TNT in most cases because of its high absorptivity at the excitation wavelength.15 To increase the selectivity toward TNT, it is interesting to include a binding site for nitrotoluene moiety. We selected calix[4]arene scaffolds because of its pre-organized three-dimensional structures with suitable hydrophobic cavity. Recently, calix[4]arene derivatives have been used for fluorescence detection of TNT and other nitroaromatic compounds. Lee et al. have prepared dipyrenylcalix[4]arene as fluorescence-based chemosensor for trinitroaromatics in acetonitrile and chloroform.16 Costa et al. have reported phenylene-ethynylene π-conjugated system containing calix[4]arene receptor for detecting nitroaromatic compounds.17 In 2013, Kandpal et al. have reported the upper-rim modification of calix[4]arene with benzimidazole moiety that efficiently served as a TNT receptor both in solution and solid state.18 The cavity of calix[4]arene has been reported to accommodate various aromatic and electron deficient compounds including toluene19 and nitromethane.20 Most recently, Cao et al. have prepared a deep cavity tetranaphthyl-calix[4]arene which exhibited selectivity toward p-nitrophenol in acetonitrile.21 In our molecular design, the upper-rim of calix[4]arene is extended with a π-conjugated system of phenylacetylene to produce a wider cavity for entrapping TNT. The sensing study in high water content media is particularly emphasized here as it is more relevant to real environmental samples. We hypothesized that the more hydrophilic TNP would be less likely to be included into the hydrophobic cavity of calix[4]arene in aqueous medium. However hydrophilic groups such as carboxyl, hydroxyl and amino groups were placed on the modified wider rim to ensure sufficient solubility in aqueous media (Fig. 1). The placement of these groups should also provide different electronically push–pull effects in connection with the electron donating alkoxy groups on the other rim, the narrow rim.
Experimental
General information
All reagents were purchased from Sigma-Aldrich, Fluka® (Switzerland) and Merck® (Germany). Organic solvents for reaction work up and chromatography were commercial grades, which were distilled prior to use. Thin layer chromatography (TLC) was performed on silica gel coated aluminum sheets (Merck Kieselgel 60 F254) (Merck KgaA, Darmstadt, Germany). Column chromatography was performed on silica gel (Merck Kieselgel 60G) (Merck KgaA, Darmstadt, Germany).Synthesis of compound 2
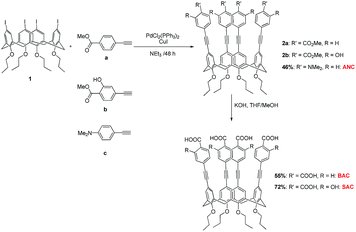
A typical synthesis of compound 2 (ref. 22) was performed by mixing PdCl2(PPh3)2 (40 mol%) and CuI (40 mol%) with 5,11,17,23-tetraiodo-25,26,27,28-tetra-(n-propoxy)calix[4]arene 1 (ref. 23) (0.1 mmol) in dry and degassed triethylamine (20 mL) at room temperature for 30 min followed by an addition of a 4-ethynylbenzene derivative (0.8 mmol). The stirring was continued under nitrogen for 48 h before the solvent was removed by a rotating evaporator. The residue was dissolved in ethylacetate (100 mL) and extracted with water (100 mL × 3). The organic layer was dried over anhydrous MgSO4, filtered and evaporated to provide a crude product which was purified by column chromatography on silica gel.Hydrolysis of ester 2
Electrochemical measurements
Cyclic voltammetry (CV) measurements were carried out in a three-electrode system consisting of Ag/Ag+ (0.01 M AgNO3) as the reference electrode, glassy carbon as the working electrode and the platinum-wire as the counter electrode using a scan rate of 50 mV s−1 under nitrogen atmosphere to find HOMO levels of the fluorophores (Fig. S2†). Ferrocene was used as the external standard for calibration CV curves. All samples and the external standard were dissolved in the supporting electrolyte (0.1 M of tetra-n-butylammonium hexafluorophosphate in anhydrous dimethylformamide) to give final concentrations of 1 mM.The HOMO energy levels of the fluorophores were calculated from the following equations: EHOMO = −[Eonsetox − E1/2 + 4.8] eV, in which Eonsetox is the onset oxidation potential relative to the Ag/AgCl reference electrode. The half-wave potential of the ferrocene/ferrocenium (E1/2) was measured as the average of the anodic and cathodic peak potentials. The LUMO energy levels were calculated from ELUMO = EHOMO + Egap determined from 1240/λcut off in which the cut off wavelength (λcut off) is the longest wavelength showing the absorption.
Preparation of ANC fluorescent paper sensor
A filter paper strip (Whatman No. 1, 4 cm × 1.1 cm2) was immerged in a CH2Cl2 solution of ANC 1 mM for 1 min. After removal from the solution, the coated filter paper was allowed to dry in the air at room temperature for 2 hours.For the TNT vapor sensing, 0.50 g of TNT solid was placed in a 1 mL Eppendorf, covered with cotton gauze and cap-closed overnight to maintain a constant saturation vapor pressure before the ANC fluorescent paper sensor was placed on top of the cotton in the Eppendorf and then cap-closed again for 5 and 10 minutes. The quenching results were recorded by a commercial digital camera.
Results and discussion
The key step in the synthesis of the fluorophores (BAC, SAC, and ANC) was the Sonogashira cross coupling reaction between tetraiodo-calix[4]arene 1 and the ethynylbenzene derivative (i.e. methyl 4-ethynylbenzoate or methyl 4-ethynylsalixylate or N,N-dimethyl-4-ethynylaniline) according to Fig. 2 to afford 2a, 2b, and ANC in moderate yield. The base-catalyzed hydrolysis of ester 2a and 2b readily gave BAC and SAC, respectively. The 1H NMR revealed that all fluorophores were obtained in cone conformation by having the characteristic doublet signals of Hexo and Hendo of the bridged methylene protons (ArCH2Ar) around 4.4 and 3.2 ppm.The normalized electronic absorption and emission spectra of the fluorophore solutions in THF are depicted in Fig. 3 and their photophysical data are presented in Table S1.† The UV-vis absorption spectrum of each fluorophore showed a broad absorption band with λmax around 310–315 nm associated with the π–π* electronic transition of the substituted diphenylacetylene conjugated system. The similar λmax values of these fluorophores indicate their comparable electronic energy band gap. The emission spectra of SAC and BAC appeared at lower energy indicating a larger Stokes shifts comparing with that of ANC. These results may be attributed to the fact that SAC and BAC contain both electron donor and electron acceptor substituents allowing for an intramolecular charge transfer (ICT).
The HOMO and LUMO electronic energy levels of the fluorophores were summarized in Table S1.† Their LUMO energy levels in the range of −2.18 to −1.54 eV are clearly higher than those of TNT, DNT and PA (Fig. S1†) that allow for an electron transfer from the excited fluorophores to TNT, DNT and PA in the PET process.
The response of ANC, BAC and SAC to various electron deficient aromatic compounds such as (2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), picric acid (PA), 2,4-dinitrophenol (DNP), 2-nitrophenol (2-NP), 3-nitrophenol (3-NP), 4-nitrophenol (4-NP), 4-nitrobenzoic acid (4-NBA), benzoic acid (BA), and 2-chlorobenzoic acid (2-ClBA)) in aqueous solutions were studied (Fig. 4). The fluorescence signals of SAC and BAC were quenched by about 2 times or less by the electron deficient aromatic compounds. On the other hand, the fluorescence signal of ANC was selectively quenched by TNT (∼8 times) and DNT (∼4 times). As designed, the quenching effect of PA on ANC was also much lesser than TNT and DNT owing to the more hydrophilicity of PA. It is also important to note that the electron donating amino groups on the phenyl ring on ANC enhances the sensitivity toward TNT comparing with BAC and SAC. The results suggest that TNT molecule interact with the phenyl rings on the wider rim of the fluorophore.
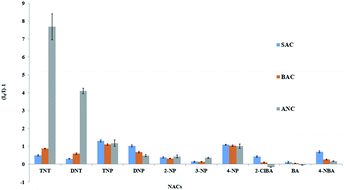 | ||
| Fig. 4 Fluorescence quenching effects of various electron deficient aromatic compounds (50 μM) on the fluorophores (0.5 μM) in 1% THF/H2O. | ||
The Stern–Volmer plots of the fluorescence intensity ratio (I0/I) of ANC against the TNT concentration as shown in Fig. 5 gave the Stern–Volmer constant (Ksv) of 1.09 × 105 M−1. The plot also showed a very good linearity in the range of 0–10 μM TNT with the limit of detection (LOD) of 0.3 μM (68 ppb) which is lower than TNT level typically found in ground water and soil sites near a munitions plant (LOD at the three times noise = 3σ/K where σ is the standard deviation of the blank measurements, and K is the slope of the calibration curve). Meanwhile, the Ksv constant for DNT and PA was found to be 5.97 × 104 M−1 and 2.09 × 104 M−1 respectively. Fig. 6 shows Stern–Volmer plots at 25 and 50 °C with the Ksv values of 1.09 × 105 M−1 and 5.08 × 104 M−1, respectively (Fig. S3†). The decrease in Ksv values with the increase in temperature supports a static quenching mechanism.24
 | ||
| Fig. 5 The fluorescence quenching ratio of ANC upon the addition of TNT (left). Stern–Volmer plots for fluorescence quenching of the fluorophores with TNT (right). | ||
The interaction between ANC and TNT were further examined by 1H NMR titration (Fig. 7). Upon increasing amount of TNT added to ANC solution, the aromatic proton signals (b and c) of the phenyl ring at the wider rim gradually upfield shifted, whereas proton signals of the phenyl ring at the narrower rim showed no significant shifts confirming the interaction of TNT with the aniline rings at the extended wider rim of calix[4]arene, probably via an insertion into the ANC cavity (Fig. 8).
 | ||
| Fig. 7 1H NMR of ANC in CDCl3 in the absence and presence of TNT (left). Chemical shift change (Δλ) plot (right). | ||
On the basis of Job plot, the complex ratio between ANC and TNT obtained from emission data showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complexation (Fig. S6†). This interaction is consistent with the nonradiative PET from the LUMO energy level of the electron rich ANC in its excited state to the LUMO of the electron poor TNT.
1 stoichiometric complexation (Fig. S6†). This interaction is consistent with the nonradiative PET from the LUMO energy level of the electron rich ANC in its excited state to the LUMO of the electron poor TNT.
The pH-dependent titration of ANC was investigated in the pH range of 3–10. There was no significant change of the intensity as shown in Fig. S5† indicating that the protonation at dimethyl amine system on ANC did not affect the fluorescence of ANC. Furthermore, we also compared the quenching of fluorescence of ANC to TNT in DMF with aqueous solution (1% THF) (Fig. S7†). The quenching in aqueous solution (∼90%) was much higher than in DMF (∼20%). The result showed that water can forced TNT to insert into ANC cavity.
We further carried out TNT detection both in vapor mode and contact mode by using paper strips. Upon exposure to TNT vapor (8.02 × 10−6 mm Hg at 25 °C), the fluorescence spot on a filter paper strip was quenched (Fig. 9, left). In contact mode test, the glove-wearing thumb, rubbed with TNT was pressed on a paper strip. Under UV light, the dark finger print of the thumb appeared (Fig. 9, right). These results demonstrated that the paper strips coated with ANC may be applied for a visual on-site detection of trace residues of TNT and its vapor.
Conclusions
The fluorescent arylethynyl calix[4]arenes were successfully synthesized. With aniline rings on the wider rim, ANC exhibited high sensitivity and selectivity of fluorescence quenching by TNT in aqueous medium. The electron rich aniline rings provide high sensitivity while the size and hydrophobicity of the cavity of the modified calix[4]arene provide high selectivity as evidenced by the shift of 1H NMR signals. The Stern–Volmer constant for TNT fluorescence quenching was 1.09 × 105 M−1 with the detection limit of 0.3 μM (68 ppb). In addition, the filter paper strips coated with ANC are potentially useful for visual detection of trace residues of TNT residue and its vapor.Acknowledgements
We would like to thank The Thailand Research Fund the Royal Golden Jubilee Ph.D. Program (Grant no. PHD/0234/2552), Nanotechnology Center (NANOTEC), though its program of Center of Excellence Network, National Research University of CHE and the Ratchadaphiseksomphot Endowment Fund (AM1006A) for financial support and student scholarships. This work is part of the Project for Establishment of Comprehensive Center for Innovative Food, Health Products and Agriculture supported by the Thai Government Stimulus Package 2 (TKK2555, SP2) and the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (RES560530126-AM). Y. Y. was supported by the 90th Anniversary of Chulalongkorn University scholarship and N. N. would also like to acknowledge the financial supports from the Thailand Research Fund (TRF-MRG5680031) and KMUTT Research Fund.Notes and references
- (a) US Department of Health and Human Services, Toxicological profile for 2,4,6-trinitrotoluene, Public Health Service, Agency for Toxic Substances and Disease Registry, 1995 Search PubMed; (b) D. Ownby, J. Belden, G. Lotufo and M. Lydy, Chemosphere, 2005, 58, 1153 CrossRef CAS PubMed.
- (a) F. Fant, A. Sloovere, K. Matthijsen, C. Marle, S. Fantroussi and W. Verstraete, Environ. Pollut., 2000, 111, 503 CrossRef; (b) Environmental Protection Agency, Innovative treatment technologies: Annual status report, 8th edn, 1996 Search PubMed.
- (a) S. Toal and W. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC; (b) A. Rose, Z. G. Zhu, C. F. Madigan, T. M. Swager and V. Bulovic, Nature, 2005, 434, 876 CrossRef CAS PubMed; (c) M. B. Pushkarsky, I. G. Dunayevskiy, M. Prasanna, A. G. Tsekoun, R. Go and C. K. N. Patel, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 19630 CrossRef CAS PubMed; (d) S. J. Toal, D. Magde and W. C. Trogler, Chem. Commun., 2005, 40, 5465 RSC; (e) L. Shriver-Lake, B. Donner and F. Ligler, Environ. Sci. Technol., 1997, 31, 837 CrossRef CAS; (f) H. Sohn, R. M. Calhoun, M. J. Sailor and W. C. Trogler, Angew. Chem., Int. Ed., 2001, 40, 2104 CrossRef CAS.
- G. He, N. Yan, H. Wang, L. Ding, S. Yin and Y. Fang, Macromolecules, 2011, 44, 4759 CrossRef CAS , and reference therein.
- (a) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS PubMed; (b) K. J. Albert, N. S. Lewis, C. L. Schauer, G. A. Sotzing, S. E. Stitzel, T. P. Vaid and D. R. Walt, Chem. Rev., 2000, 100, 2595 CrossRef CAS PubMed; (c) T. Naddo, Y. Che, W. Zhang, K. Balakrishnan, X. M. Yang, M. Yen, J. C. Zhao, J. S. Moore and L. Zang, J. Am. Chem. Soc., 2007, 129, 6978 CrossRef CAS PubMed.
- (a) S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef PubMed; (b) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS PubMed; (c) J. S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 5321 CrossRef CAS; (d) J. S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef CAS.
- (a) S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC; (b) H. Sohn, R. M. Calhoun, M. J. Sailor and W. C. Trogler, Angew. Chem., Int. Ed., 2001, 40, 2104 CrossRef CAS; (c) H. Sohn, M. J. Sailor, D. Magde and W. C. Trogler, J. Am. Chem. Soc., 2003, 125, 3821 CrossRef CAS PubMed; (d) J. C. Sanchez, A. G. DiPasquale, A. L. Rheingold and W. C. Trogler, Chem. Mater., 2007, 19, 6459 CrossRef CAS.
- (a) L. Chen, D. McBranch, R. Wang and D. Whitten, Chem. Phys. Lett., 2000, 330, 27 CrossRef CAS; (b) C. P. Chang, C. Y. Chao, J. H. Huang, A. K. Li, C. S. Hsu, B. R. Lin and A. C. Su, Synth. Met., 2004, 144, 297 CrossRef CAS PubMed.
- Y. L. Liu, R. C. Mills, J. M. Boncella and K. S. Schanze, Langmuir, 2001, 17, 7452 CrossRef CAS.
- (a) M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543 RSC; (b) M. S. Meaney and V. L. McGuffin, Anal. Bioanal. Chem., 2008, 391, 2557 CrossRef CAS PubMed.
- Q. Zhou and T. M. Swager, J. Am. Chem. Soc., 1995, 117, 12593 CrossRef CAS.
- S. Kumar, N. Venkatramaiah and S. Patil, J. Phys. Chem. C, 2013, 117, 7236 CAS.
- (a) M. E. Germain, T. R. Vargo, B. A. McClure, J. J. Rack, P. G. V. Patten, M. Odai and M. J. Knapp, Inorg. Chem., 2008, 47, 6203 CrossRef CAS PubMed; (b) M. S. Meaney and V. L. McGuffin, Anal. Bioanal. Chem., 2008, 391, 2557 CrossRef CAS PubMed; (c) M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543 RSC; (d) G. V. Zyryanov and M. A. Palacios, Org. Lett., 2008, 10, 3681 CrossRef CAS PubMed; (e) Y. H. Lee, H. Liu, J. Y. Lee, S. H. Kim, S. K. Kim, J. L. Seeler, Y. Kim and J. S. Kim, Chem.–Eur. J., 2010, 16, 5895 CrossRef CAS PubMed; (f) Y. Peng, A. J. Zhang, M. Dong and Y. W. Wang, Chem. Commun., 2011, 47, 4504 Search PubMed; (g) B. Gole, S. Shanmugaraju, A. K. Bar and P. S. Mukherjee, Chem. Commun., 2011, 47, 10046 RSC; (h) M. Kumar, V. Vil and V. Bhalla, Langmuir, 2012, 28, 12417 CrossRef CAS PubMed; (i) K. Park, J. Yoo, J. W. Ka and C. H. Lee, Bull. Korean Chem. Soc., 2012, 33, 675 CrossRef CAS; (j) N. Niamnont, N. Kimpitak, K. Wongravee, P. Rashatasakhon, K. K. Baldridge, J. S. Siegel and M. Sukwattanasinitt, Chem. Commun., 2013, 49, 780 RSC; (k) S. Shanmugaraju, H. Jadhav, R. Karthik and P. S. Mukherjee, RSC Adv., 2013, 3, 4940 RSC; (l) S. Shanmugaraju, S. A. Joshi and P. S. Mukherjee, J. Mater. Chem., 2011, 21, 9130 RSC.
- B. Xu, X. Wu, H. Li, H. Tong and L. Wang, Macromolecules, 2011, 44, 5089 CrossRef CAS.
- Y. Ma, H. Li, S. Peng and L. Wang, Anal. Chem., 2012, 84, 8415 CrossRef CAS PubMed.
- Y. H. Lee, H. Liu, J. Y. Lee, S. H. Kim, S. K. Kim, J. L. Sessler, Y. Kim and J. S. Kim, Chem.–Eur. J., 2010, 16, 5895 CrossRef CAS PubMed.
- (a) A. I. Costa and J. V. Prata, Sens. Actuators, B, 2012, 161, 251 CrossRef CAS PubMed; (b) A. I. Costa, H. D. Pinto, L. F. V. Ferreira and J. V. Prata, Sens. Actuators, B, 2012, 161, 702 CrossRef CAS PubMed.
- M. Kandpal, A. K. Bandela, V. K. Hinge, V. R. Rao and C. P. Rao, ACS Appl. Mater. Interfaces, 2013, 5, 13448 CAS.
- L. Motta, J. B. Regnouf De Vains, C. Bavoux and M. Perrin, J. Chem. Crystallogr., 1995, 25, 401 CrossRef CAS.
- R. Rojanathanes, T. Tuntulani, W. Bhanthumnavin and M. Sukwattanasinitt, Org. Lett., 2005, 7, 3401 CrossRef CAS PubMed.
- X. Cao, D. Tian, F. Miao, F. Zhang, L. Luo and H. Li, Tetrahedron Lett., 2014, 55, 2029 CrossRef CAS PubMed.
- (a) N. Armaroli, G. Accorsi, Y. Rio, P. Ceroni, V. Vicinelli, R. Welter, T. Gu, M. Saddik, M. Hollerd and J.-F. Nierengarten, New J. Chem., 2004, 28, 1627 RSC; (b) G. Hennrich, M. T. Murillo, P. Prados, K. Song, I. Asselberghs, K. Clays, A. Persoons, J. B. Buchholzc and J. Mendozac, Chem. Commun., 2005, 40, 2747 RSC.
- B. Klenke and W. Friedrichsen, J. Chem. Soc., Perkin Trans. 2, 1998, 4, 3377 RSC.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, John Wiley & Sons, Inc., Kluwer, 3rd edn, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and spectroscopic data. See DOI: 10.1039/c5ra02758f |
| This journal is © The Royal Society of Chemistry 2015 |