The effect of light and humidity on the stability of silver nanowire transparent electrodes†
Abstract
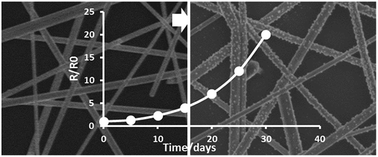
Transparent electrodes based on silver nanowire (AgNW) films have attracted considerable attention owing to their high electrical and thermal conductivity, optical transparency, and flexibility. However, the long-term reliability of AgNW electrodes, has seldom been studied. In this work, the effects of storage environment, such as, natural light and humidity, on the long-term reliability have been investigated in detail. The increase of electrical resistance with storage time greatly depended on the natural light illumination, especially ultraviolet (UV) illumination, and high humidity seriously accelerated the failure of AgNW electrodes. All the results were dependent on the storage temperature. Some over-coating layers have been used to protect the AgNW electrodes from light and humidity in environment and the results showed that an epoxy resin protecting layer exhibited the longest lifetime: over 40 days at 85 °C and 85% relative humidity.

 Please wait while we load your content...
Please wait while we load your content...