Evaluation of the Yasuda parameter for the atmospheric plasma deposition of allyl methacrylate
Abstract
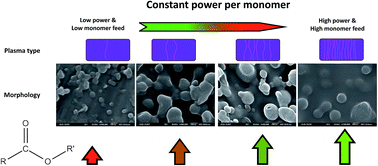
This work studies the influence of the proportional change in discharge power and the monomer feed on the morphology and the chemistry of atmospheric plasma deposited films. Atmospheric plasma coatings of allyl methacrylate were deposited using dielectric barrier discharge plasma under different conditions but always under the same ratio between the discharge power and monomer feed (W/FM). It is shown that a constant W/FM does not necessarily provide the same chemistry and the same morphology for atmospheric pressure plasma. This is explained by the higher discharge power of the plasma resulting in an increase of streamers which alter the distribution of energy among the plasma species. On the surface of the deposited coatings, globular-like features were observed, which are suggested to be formed in the volume of the discharge. The deposition rate is also influenced. providing thicker coatings, when high monomer feed/high power are used. Finally, infrared spectra showed a higher retention of the ester functionality at high power/high monomer feed.


 Please wait while we load your content...
Please wait while we load your content...