Fabrication and characterization of a novel nanofiltration membrane by the interfacial polymerization of 1,4-diaminocyclohexane (DCH) and trimesoyl chloride (TMC)
Gui-E. Chen*a,
Yan-Jun Liua,
Zhen-Liang Xub,
Yong-Jian Tangb,
Hui-Hong Huanga and
Li Suna
aSchool of Chemical and Environmental Engineering, Shanghai Institute of Technology, 100 Haiquan Road, Shanghai 201418, China. E-mail: chenguie@sit.edu.cn; Fax: +86-21-64941192; Tel: +86-21-64941192
bState Key Laboratory of Chemical Engineering, Membrane Science and Engineering R&D Lab, Chemical Engineering Research Center, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
First published on 7th April 2015
Abstract
This study focuses on the preparation and nanofiltration properties of a novel thin-film composite polyamide membrane formed by the interfacial polymerization of 1,4-diaminocyclohexane (DCH) and trimesoyl chloride (TMC) on a porous polysulfone supporting membrane. At the same time, we found that the introduction of sodium N-cyclohexylsulfamate (SCHS) can improve to a degree the water flux and salt rejection. The active surface of the membrane was characterized by employing SEM and AFM. The performance of the nanofiltration membrane was optimized by considering the preparation conditions, including the monomer concentration, reaction time, curing conditions and SCHS concentration. The resulting NF membrane prepared under the optimum conditions exhibited a Na2SO4 rejection of 98.1% and a water flux of 44.6 L m−2 h−1 at 0.6 MPa. The pore size of the NF membrane was about 0.33–0.42 nm, which was calculated from the rejection of PEG and carbohydrates, respectively.
1. Introduction
With the current progress of urbanization and industrialization in the world, the scarcity of potable water has become an alarming question, especially in arid regions. To deal with water scarcity, many efforts have been made to remove heavy metals before its discharge and reuse wastewater. Nanofiltration (NF), as an important and environment-friendly separation technique between ultrafiltration and reverse osmosis, has attracted more and more research attention due to its low energy consumption,1 higher rejection of multivalent salts and compounds with molecular weight >300, and broad applications in the desalination of brackish water and seawater,2 wastewater treatment,3 and industrial substance separation,4 etc. Therefore, nanofiltration membranes with good separation performance and other excellent properties are required for more complicated applications.At present, among the different successful NF membrane preparation techniques, interfacial polymerization is of particular interest because the selective layer and the porous support layer can be optimized separately.5,6 Though the number of applications for NF by interfacial polymerization is increasing steadily, this technology still suffers from some drawbacks, such as membrane fouling and insufficient separation factor.7 Compared with membrane modification improvement, it is more accessible to ameliorate NF performance by finding a novel monomer and adding it to an outstanding additive. To address different separation requirements, a series of negatively charged nanofiltration membranes were prepared by exploiting new monomers or adding a new additive with special functional groups. These new monomers include polyhexamethylene guanidine hydrochloride,8 tannins,9 polyvinylamine,10 N-aminoethyl piperazine propane sulfonate and PIP mixtures,11 2,2′-bis(1-hydroxyl-1-trifluoromethyl-2,2,2-trifluoroethyl)-4,4′-methylene-dianiline,12 disulfonated bis[4-(3-aminophenoxy)phenyl]sulfone,13 etc. Some of the additives are the following: silica,12 poly(styrene sulfonic acid) sodium salt,14 TiO2, Al2O3, ZrO2, Al2O3 and TiO2 mixtures,15 etc. Among all the preparation methods, there are few reports of aliphatic cyclic diamine as a monomer or amine salt as an additive.
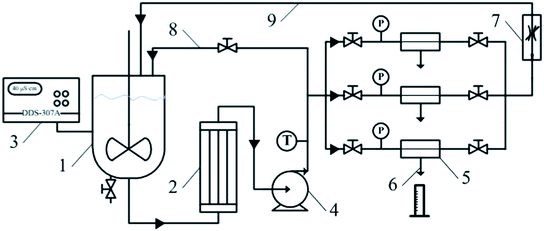
In order to improve nanofiltration membrane performance, such as salt rejection, water flux and antifouling performance, information on the inherent material properties and surface structures of the active layer polymers is necessary. In this paper, we chose 1,4-diaminocyclohexane as a water-soluble monomer to prepare a novel NF membrane, considering that aliphatic amines have anti-fouling ability. Additionally, sodium N-cyclohexylsulfamate (SCHS) dissolved in the aqueous phase was introduced as the additive during the interfacial polymerization process. The resulting NF membranes were measured by a cross-flow nanofiltration system (Fig. 2), demonstrating their great potential. Remarkably, as an inorganic salt with a negative charge in solution, SCHS could not only improve the hydrophilicity and flux of the membrane, but also could enhance the anti-fouling properties of the membrane. In this work, the effects of different polymerization conditions on membrane performance were investigated, and the NF membrane performances were evaluated using different methods.
2. Experimental
2.1. Materials
1,4-Diaminocyclohexane (DCH, purity > 98%) was purchased from Tansoole; trimesoyl chloride (TMC, purity > 98%) was bought from Qingdao Ocean Chemical Company. Their chemical structures are shown in Fig. 1. | ||
| Fig. 1 Chemical structures of the monomers used: 1,4-diaminocyclohexane (DCH) and trimesoyl chloride (TMC). | ||
Polysulfone ultrafiltration (PSF-UF) supporting membranes (MWCO = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) were fabricated in our lab. Sodium N-cyclohexylsulfamate (SCHS) was purchased from Tansoole; other reagents were purchased from the Sinopharm Chemical Reagent company, which were of analytical grade purity and used without further purification.
000 Da) were fabricated in our lab. Sodium N-cyclohexylsulfamate (SCHS) was purchased from Tansoole; other reagents were purchased from the Sinopharm Chemical Reagent company, which were of analytical grade purity and used without further purification.
2.2. Preparation of composite nanofiltration membranes
The composite NF membranes were fabricated by an interfacial polymerization technique. PES ultrafiltration membranes were used as the porous support. The PES ultrafiltration membranes were washed thoroughly with water over 24 h before use as the porous support. Aqueous solutions of DCH and n-hexane solutions of TMC at different concentrations were prepared. The PSF membranes were first soaked in aqueous solutions for about 5 min to ensure that DCH monomers could diffuse into the porous support. The residual water on the surface was drained off by an air knife. Subsequently, the organic phase was poured on the top of the PSF-UF membrane for a predetermined time (10–60 s) for interfacial polymerization. The excess organic solution was removed from the surface, and the coated surfaces were air-dried in an oven at a certain temperature (post treatment temperature) and for a certain time (post treatment reaction time) for further polymerization reaction and hexane evaporation. Finally, the composite NF membranes were rinsed with deionized water and stored in 1% NaHSO3 solution until they were tested.2.3. Characterization of composite nanofiltration membranes
The membrane samples used for ATR-FTIR, SEM and AFM analysis were rinsed with de-ionized water several times and dried under a vacuum at 40 °C for 24 h.The chemical structures of the TFC (thin-film composite) membranes were characterized using ATR-FTIR (Perkin-Elmer Spectrum 2000 FTIR spectrometer) to confirm the existence of SCHS in the membrane and the interfacial polymerization reactions.
Scanning electron microscopy (SEM, S-3400N, Hitachi) was used to analyze the surface and cross-sectional morphologies of the composite NF membranes. The membranes were fractured in liquid nitrogen to obtain clean cuts for cross sectional view. The samples were then gold sputtered for producing electrical conductivity.
Atomic force microscopy (AFM, Veeco, NanoScope III a Multimode AFM) was used to analyze the surface morphology and roughness of prepared membranes. The membrane surfaces were imaged with a scan size of 5 μm × 5 μm. The surface roughness was reported in terms of root mean square (RMS).
A contact angle measurement instrument (JC2000D, PowerEach, China) was utilized to determine the water contact angle of the NF membranes.
2.4. Permeability, rejection, pore size
The separation performance tests of the thin-film composite membranes were carried out at 0.6 MPa and 25.0 °C employing a cross-flow nanofiltration system. The system (Fig. 2) used for filtration contains a membrane cell, plunger pump, pressure gauge and solution vessel. Membranes with an effective area of 75 cm2 were loaded into the cell for filtration. The feed for the permeation test was de-ionized water or de-ionized water with added solutes such as Na2SO4, MgSO4, MgCl2, NaCl, or polyethylene glycol (PEG) of different molecular weights. The permeability F was calculated using the equation:where V (L) is the total volume of the permeate collected under a transmembrane pressure of 0.6 MPa at the time t (h) and A is the effective area of the membrane (m2). The solute rejection rate (R) was calculated using the equation:
where Cp and Cf are the solute concentrations in the permeate and feed solutions, respectively. The ion concentrations were measured using a DDS-11A conductance meter (Shanghai Neici Instrument Company). The salt rejection rate was calculated using the conductivity–concentration curves. The concentrations of organics were determined using a TOC analyzer (TOC-VCPH, SHIMADZU, JAPAN). The results presented are average data with standard deviation from at least three samples of each type of membrane.
The pore size, pore size distribution and molecular weight cut-off (MWCO) of the SCHS/DCH–TMC NF membrane were calculated through two series permeation tests, of which one test used a group of five PEG with molecular weights of 200, 400, 600, 1000 and 2000 Da as model solutes16 and the other test used glucose, sucrose and raffinose as model solutes. The pore size distribution was calculated with the assumption of no gap and hydrodynamic interactions between the membrane material and the organic solutes. The MWCO value was taken as the molecular weight of the solute at which the membrane rejection rate was 90% . The mean effective pore radius of the membrane (rp) was assumed to be the same as the geometric mean radius of the solute (rs) when R equals 50%. The geometric standard deviation of the membrane (σp) was assumed to be the geometric standard deviation (σg), which is the ratio of the Stokes radius when R equals 84.13 to the Stokes radius when R equals 50%. Based on the hypothesis, the pore size distribution of membrane can be expressed as the probability density function17–22
The Stokes radii of the organic solutes used during the pore size distribution tests can be calculated from the following formulas:18,24
| For PEG, rp = 16.73 × 10−12 × M0.557 |
For small molecules, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rp = −1.4962 + 0.4654 rp = −1.4962 + 0.4654![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M |
2.5. Anti-biofouling performance assessment of the composite nanofiltration membranes
In the antifouling experiment, BSA was chosen as a representative protein in natural water sources to evaluate the antifouling properties of the NF membranes. 500 mg L−1 BSA was forced to permeate through the membrane under an operation pressure of 0.6 MPa, and the flux was recorded as Jw. The antifouling experiment was carried out over a time period of 24 h. To analyze the antifouling properties in detail, several ratios were defined.11,25–27 The flux decay ratio (DR) was calculated as follows:where Jw0 and Jwt are the fluxes at the initial time and time t of the antifouling test, respectively. A lower DR value means better antifouling properties of the nanofiltration membrane, corresponding to only slight deposition or adsorption of fouling on the membrane surface. After the flux decay measurements, the solutions were then poured out and water was added to the filtration cell. The used nanofiltration membranes were cleaned directly in the cell for 30 min under magnetic stirring. Lastly, the cell was emptied and refilled with water again. The water flux (Jw2) of the cleaned membrane was measured. The flux recovery ratio (FRR) was calculated as follows:
The higher the FRR value, the better the antifouling properties of the nanofiltration membrane.
2.6. Long-term stability test of the composite nanofiltration membranes
A long-term test was conducted at a pressure of 0.6 MPa with a 2000 ppm Na2SO4 aqueous solution at pH 7.0 and 25.0 °C to investigate the durability and performance stability of the NF membrane. Periodical measurements were carried out to check the water flux and salt rejection of the membrane.3. Results and discussion
3.1. Chemical structures of the membrane surface
The FTIR spectra of the DCH and SCHS samples and the ATR-FTIR spectra of the PSF support membrane, DCH–TMC composite membrane and SCHS/DCH–TMC composite membrane are presented in Fig. 3 to analyze the chemical structural changes of the composite membrane. In addition to the typical PSF bands of the substrate, the DCH–TMC composite membrane and SCHS/DCH–TMC composite membranes possessed additional peaks at 1651 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch), 1619 cm−1 (N–H stretch) and 1434 cm−1 (C–N stretch) that corresponded to the amide group.28–30 It can be seen from the figure that interfacial polymerization between DCH and TMC occurred and a polyamide active layer was formed. Moreover, compared with the DCH–TMC composite membrane, the SCHS/DCH–TMC composite membrane exhibited new peaks at 1111 and 951 cm−1, which are the characteristic absorbances of the S
O stretch), 1619 cm−1 (N–H stretch) and 1434 cm−1 (C–N stretch) that corresponded to the amide group.28–30 It can be seen from the figure that interfacial polymerization between DCH and TMC occurred and a polyamide active layer was formed. Moreover, compared with the DCH–TMC composite membrane, the SCHS/DCH–TMC composite membrane exhibited new peaks at 1111 and 951 cm−1, which are the characteristic absorbances of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and S–N bonds of the sulfonamide group.31 Based on the results of the ATR-FTIR analysis, the chemical structure of the active skin layer formed through the reaction of TMC with DCH and SCHS is shown schematically in Fig. 4, which also gives the formula for the polymerization step.
O and S–N bonds of the sulfonamide group.31 Based on the results of the ATR-FTIR analysis, the chemical structure of the active skin layer formed through the reaction of TMC with DCH and SCHS is shown schematically in Fig. 4, which also gives the formula for the polymerization step.
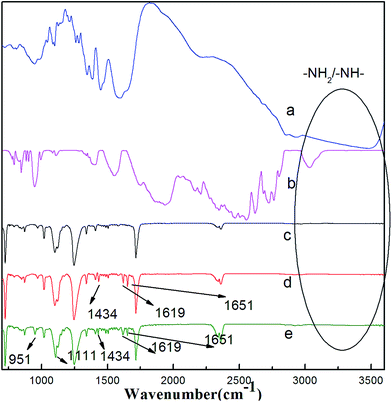 | ||
| Fig. 3 FTIR spectra of (a) DCH and (b) SCHS, ATR-FTIR spectra of (c) the PSF support membrane; (d) the DCH–TMC composite membrane and (e) the SCHS/DCH–TMC composite membrane. | ||
 | ||
| Fig. 4 Structure of the polyamide skin layer formed by the interfacial polymerization of CHD with TMC and SCHS. | ||
3.2. Analysis of membrane morphology
The structure and surface roughness of the membranes were characterized by using SEM and AFM, respectively (Fig. 5 and Fig. 6). The surface and cross-section morphologies of the membranes were visualized by SEM. It can be seen clearly that the DCH–TMC composite membrane takes on a composite structure, namely a thin and dense active functional layer existing on the porous polysulfone supporting membrane, and that adding SCHS results in an increase in the skin layer density of the resulting membrane. After further measurement, the thickness of the dense layer was found to be about 200 nm. Quantitative analysis of the roughness of the surface area was made possible by AFM image statistics. The average roughness (RMS) is defined as the mean of the root of the deviation from the standard surface.23 In Fig. 6, the RMS of various membranes are as follows: polysulfone support membrane is 5.57 nm, DCH–TMC composite membrane is 227.34 nm, and the SCHS/DCH–TMC composite membrane is 128.37 nm, which is in good agreement with the SEM results.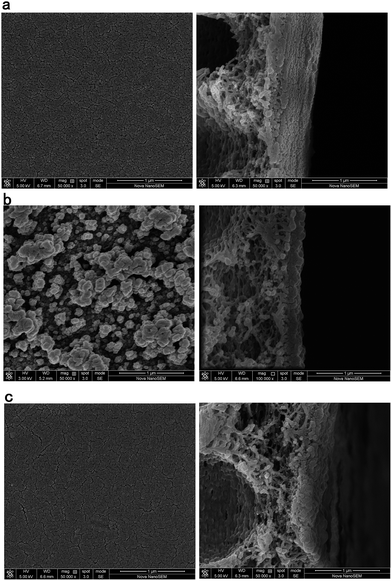 | ||
| Fig. 5 SEM images of the membranes (left: surface; right: cross section): (a) polysulfone support membrane, (b) DCH–TMC composite membrane, (c) SCHS/DCH–TMC composite membrane. | ||
 | ||
| Fig. 6 AFM images of the surface morphologies of the (a) polysulfone support membrane, (b) DCH–TMC composite membrane and (c) SCHS/DCH–TMC composite membrane. | ||
3.3. Effects of preparation conditions on filtration performance of the nanofiltration membranes
It’s well-known that the performance of the composite nanofiltration membranes is determined by the chemistry and the preparation conditions of the thin selective layer.32–38 In this section, the NF performance of DCH–TMC/PSF composite membranes prepared with variable conditions was investigated to determine the optimized fabrication parameters. | ||
| Fig. 7 Effect of DCH concentration on the salt rejection and water flux of the resulting membranes, tested with 2000 ppm Na2SO4 aqueous solution at 0.6 MPa, 25 °C and pH 7.0. | ||
It can be seen clearly from the figure that when the DCH concentration of the aqueous phase was increased from 1.0 to 2.0% (w/v) at a fixed TMC concentration of 0.15% (w/v), the salt rejection of the membranes progressively increased, while the water flux decreased. When the DCH concentration exceeded 2.0% (w/v), the salt rejection changed only slightly.
Similarly, the effect of the concentration of TMC in the organic phase was also investigated to optimize the performance of the DCH–TMC NF membrane. Fig. 8 shows the performance of the composite membranes prepared under the conditions of DCH concentration = 2.0% (w/v), reaction time = 15 s, curing temperature = 80 °C and curing time = 5 min. The salt rejection of the membranes increased at first until the TMC concentration in the organic phase reached 0.25% (w/v), and then changed only slightly, while the water flux of the membranes decreased rapidly with increasing TMC concentration to 0.50% (w/v).
 | ||
| Fig. 8 Effect of TMC concentration on the salt rejection and water flux of the resulting membranes tested with 2000 ppm Na2SO4 aqueous solution at 0.6 MPa, 25 °C and pH 7.0. | ||
This observation can be explained following the work of Freger on PA film formation kinetics and that of Nadler and Srebnik.5,39–42 The concentrations of monomers in both the aqueous and organic phases have great effects on the rate of the interfacial polymerization. The interfacial polymerization occurring between a diamine and an acid chloride takes place on the organic side of the two phase interface. When DCH and/or TMC are at low concentration, the rate of the reactions is expected to be lower and the polyamide skin layer is formed by low molecular weight polymer, which causes lower salt rejection and higher water flux. With increasing DCH concentration and/or TMC concentration, the formation of the thin barrier layer tends to reach a maximum thickness and density since the film thickness remains almost unchanged. Thus, the water flux is decreased and the salt rejection is increased. Further increase in the concentration of DCH and/or TMC, however, tends to have little effect on the rate and extent of polymerization, so the water flux and salt rejection change only slightly.
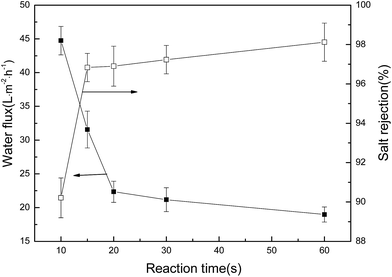 | ||
| Fig. 9 Effect of interfacial reaction time on the salt rejection and water flux of the resulting membranes tested with 2000 ppm Na2SO4 aqueous solution at 0.6 MPa, 25 °C and pH 7.0. | ||
It’s well known that the interfacial polymerization between DCH and TMC occurs on the organic side of the aqueous–organic interface, and the reaction is diffusion-controlled and exists as a self-limiting phenomenon. The reaction time plays an important role in determining the extent of polymerization, and thereby the cross-linking degree and thickness of the top skin layer as well as the resulting membrane performance.12,42–45 The thickness of the active layer of the NF membrane increases with increasing reaction time. When the thickness of the active layer is enough to prevent the DCH from diffusing from the aqueous phase into the organic phase, the top skin layer thickness will stop growing. Thus, the density of the active layer had no significant change as the reaction time was prolonged from 20 to 60 s. In this study, shorter reaction time lead to higher permeation of the water flux. As the reaction time increased, the water flux decreased and the salt rejection increased. Considering both the good salt rejection and high water flux, the reaction time of 15 s was selected as the optimum reaction time to prepare the membranes.
| Curing temperaturea (°C) | Curing timea (min) | Na2SO4 rejectionb (%) | Water fluxb (L m−2 h−1) |
|---|---|---|---|
| a Membrane preparation conditions: DCH concentration = 2.0% (w/v), TMC concentration = 0.25% (w/v), reaction time = 15 s.b Test conditions: feed = 2000 ppm Na2SO4 aqueous solution, pressure = 0.6 MPa, temperature = 25.0 °C and pH = 7.0. | |||
| 40 | 5 | 75.62 ± 1.21 | 47.08 ± 1.23 |
| 60 | 5 | 80.78 ± 1.34 | 44.57 ± 2.78 |
| 80 | 3 | 90.73 ± 0.89 | 37.81 ± 1.97 |
| 80 | 5 | 96.83 ± 0.72 | 31.56 ± 2.73 |
| 80 | 10 | 97.31 ± 1.01 | 23.43 ± 1.99 |
| 100 | 5 | 85.39 ± 2.01 | 28.56 ± 2.33 |
 | ||
| Fig. 10 Effect of SCHS concentration on the salt rejection and water flux of the resulting membranes tested with 2000 ppm Na2SO4 aqueous solution at 0.6 MPa, 25 °C and pH 7.0. | ||
 | ||
| Fig. 11 Rejection of different inorganic salts for the (A) DCH–TMC composite membrane and (B) SCHS/DCH–TMC NF membrane. | ||
Furthermore, when comparing the results for the composite membranes with other nanofiltration membranes which were described by other references, like a PAMAM/PAN membrane48 (15.3 m−2 h−1, Na2SO4 rejection 86.7%) and MPD membrane49 (water flux: 22.8 m−2 h−1, Na2SO4 rejection 95.5%), the results for the composite membrane (water flux: 44.6 m−2 h−1, salt rejection 98.1%) have a higher water flux or Na2SO4 rejection.
3.4. Separation performance of the optimized composite nanofiltration membranes
In this section, DCH–TMC/PSF NF membranes prepared with the optimized conditions were investigated to evaluate their potential applications. The optimum conditions are as follows: 2.0% (w/v) DCH and 0.07% (w/v) SCHS in the aqueous phase; 0.25% (w/v) TMC in the organic phase; reaction time of 15 s and curing temperature at 80 °C for 5 min.| Solute | MW (g mol−1) | Rejection (%) |
|---|---|---|
| PEG 200 | 200 | 64.52 ± 4.23 |
| PEG 400 | 400 | 90.69 ± 2.37 |
| PEG 600 | 600 | 94.72 ± 2.07 |
| PEG 1000 | 1000 | 97.34 ± 1.56 |
| PEG 2000 | 2000 | 97.69 ± 2.11 |
| Glucose | 180 | 63.56 ± 3.72 |
| Sucrose | 342 | 82.79 ± 2.83 |
| Raffinose | 504 | 92.50 ± 1.79 |
4. Conclusions
In this work, a simple and effective approach for a novel NF membrane has been demonstrated. Thin-film composite polyamide nanofiltration membranes were successfully prepared by an interfacial polymerization technique from DCH and TMC, which was confirmed by ATR-FTIR and SEM images and measured by a NF system (Fig. 2).The key finding is that the addition of SCHS has a significant influence on the NF performance of the resulting DCH–TMC composite membrane. The resulting NF membrane prepared under the optimum conditions exhibited a Na2SO4 rejection of 98.1% and a water flux of 44.6 L m−2 h−1 at 0.6 MPa. The rejections decrease in the order Na2SO4, MgSO4, MgCl2 and NaCl, with values of 98.1%, 92.0%, 80.6% and 27.0% respectively. The pore size of the NF membrane is about 0.33–0.42 nm, and the MWCO of the NF membrane is about 1000 Da, which was calculated with two different methods. In addition, the NF membrane also showed anti-fouling ability, hydrophilicity, and good stability.
Acknowledgements
The authors are thankful for the financial support received from the National Natural Science Foundation of China (51172144), Shanghai Union Program (LM201249), the Key Technology R&D Program of Shanghai Committee of Science and Technology in China (13521102000 and 14231201503), 2013 Year Special Project of the Development and Industrialization of New Materials of National Development and Reform Commission in China (20132548) and the Key Technology R&D Program of Jiangsu Committee of Science and Technology in China (BE2013031).References
- N. Hilal, H. Al-Zoubi, N. A. Darwish, A. W. Mohamma and M. Abu Arabi, Desalination, 2004, 170, 281–308 CrossRef CAS PubMed
.
- B. V. Bruggen and C. Vandecasteele, Environ. Pollut., 2003, 122, 435–445 CrossRef
.
- L. Shu, T. D. Waite and P. J. Bliss, Desalination, 2005, 172, 235–243 CrossRef CAS PubMed
.
- T. Gotoh, H. Iguchi and K.-I. Kikuchi, Biochem. Eng. J., 2004, 19, 165–170 CrossRef CAS PubMed
.
- N. K. Saha and S. V. Joshi, J. Membr. Sci., 2009, 342, 60–69 CrossRef CAS PubMed
.
- C. Wu, S. Zhang, D. Yang and X. Jian, J. Membr. Sci., 2009, 326, 429–434 CrossRef CAS PubMed
.
- B. Van der Bruggen, M. Mänttäri and M. Nyström, Sep. Purif. Technol., 2008, 63, 251–263 CrossRef CAS PubMed
.
- X. Li, Y. Cao, H. Yu, G. Kang, X. Jie, Z. Liu and Q. Yuan, J. Membr. Sci., 2014, 466, 82–91 CrossRef CAS PubMed
.
- Y. Zhang, Y. Su, J. Peng, X. Zhao, J. Liu, J. Zhao and Z. Jiang, J. Membr. Sci., 2013, 429, 235–242 CrossRef CAS PubMed
.
- M. Liu, Y. Zheng, S. Shuai, Q. Zhou, S. Yu and C. Gao, Desalination, 2012, 288, 98–107 CrossRef CAS PubMed
.
- Q.-F. An, W.-D. Sun, Q. Zhao, Y.-L. Ji and C.-J. Gao, J. Membr. Sci., 2013, 431, 171–179 CrossRef CAS PubMed
.
- D. Hu, Z.-L. Xu and Y.-M. Wei, Sep. Purif. Technol., 2013, 110, 31–38 CrossRef CAS PubMed
.
- W. Xie, G. M. Geise, B. D. Freeman, H.-S. Lee, G. Byun and J. E. McGrath, J. Membr. Sci., 2012, 403–404, 152–161 CrossRef CAS PubMed
.
- D. Hu, Z.-L. Xu and C. Chen, Desalination, 2012, 301, 75–81 CrossRef CAS PubMed
.
- T. V. Gestel, C. Vandecasteele and A. Buekenhoudt, J. Membr. Sci., 2002, 207, 73–89 CrossRef
.
- N. Hilal, M. Al-Abri, H. Al-Hinai and M. Abu-Arabi, Desalination, 2008, 221, 284–293 CrossRef CAS PubMed
.
- L.-B. Zhao, Z.-L. Xu, M. Liu and Y.-M. Wei, J. Membr. Sci., 2014, 454, 184–192 CrossRef CAS PubMed
.
- S. Zhang, F. Fu and T.-S. Chung, Chem. Eng. Sci., 2013, 87, 40–50 CrossRef CAS PubMed
.
- Y. Cui, H. Wang, H. Wang and T.-S. Chung, Chem. Eng. Sci., 2013, 101, 13–26 CrossRef CAS PubMed
.
- S. P. Sun, T. A. Hatton, S. Y. Chan and T.-S. Chung, J. Membr. Sci., 2012, 401–402, 152–162 CrossRef CAS PubMed
.
- P. H. H. Duong, T.-S. Chung, K. Jeyaseelan, A. Armugam, Z. Chen, J. Yang and M. Hong, J. Membr. Sci., 2012, 409–410, 34–43 CrossRef CAS PubMed
.
- G. Han, S. Zhang, X. Li, N. Widjojo and T.-S. Chung, Chem. Eng. Sci., 2012, 80, 219–231 CrossRef CAS PubMed
.
- J. Xiang, Z. Xie, M. Hoang, D. Ng and K. Zhang, J. Membr. Sci., 2014, 465, 34–40 CrossRef CAS PubMed
.
- J. Xiang, Z. L. Xie and M. Hoang, J. Membr. Sci., 2014, 465, 34–40 CrossRef CAS PubMed
.
- Y. Li, Y. Su, Y. Dong, X. Zhao, Z. Jiang, R. Zhang and J. Zhao, Desalination, 2014, 333, 59–65 CrossRef CAS PubMed
.
- W. Chen, Y. Su, J. Peng, Y. Dong, X. Zhao and Z. Jiang, Adv. Funct. Mater., 2011, 21, 191–198 CrossRef CAS PubMed
.
- P.-Y. Zhang, Z.-L. Xu, H. Yang, Y.-M. Wei, W.-Z. Wu and D.-G. Chen, Desalination, 2013, 319, 47–59 CrossRef CAS PubMed
.
- B. A. Anderson, A. Literati, B. Ball and J. Kubelka, Spectrochim. Acta, Part A, 2015, 134, 473–483 CrossRef CAS PubMed
.
- A. K. Ghosh, B.-H. Jeong, X. Huang and E. M. V. Hoek, J. Membr. Sci., 2008, 311, 34–45 CrossRef CAS PubMed
.
- S. A. Sundet, W. A. Murphey and S. B. Speck, journal of polymer science, 1959, XL, 389–397 CrossRef PubMed
.
- S. Muthu, G. Ramachandran, E. Isac Paulraj and T. Swaminathan, Spectrochim. Acta, Part A, 2014, 128, 603–613 CrossRef CAS PubMed
.
- Y. Ji, Q. An, Q. Zhao, H. Chen and C. Gao, J. Membr. Sci., 2011, 376, 254–265 CrossRef CAS PubMed
.
- M. N. A. Seman, M. Khayet and N. Hilal, J. Membr. Sci., 2010, 348, 109–116 CrossRef PubMed
.
- Y. Liu, B. He, J. Li, R. D. Sanderson, L. Li and S. Zhang, J. Membr. Sci., 2011, 373, 98–106 CrossRef CAS PubMed
.
- X.-Z. Wei, L.-P. Zhu, H.-Y. Deng, Y.-Y. Xu, B.-K. Zhu and Z.-M. Huang, J. Membr. Sci., 2008, 323, 278–287 CrossRef CAS PubMed
.
- Y.-C. Chiang, Y.-Z. Hsub, R.-C. Ruaan, C.-J. Chuang and K.-L. Tung, J. Membr. Sci., 2009, 326, 19–26 CrossRef CAS PubMed
.
- F. Yang, S. Zhang, D. Yang and X. Jian, J. Membr. Sci., 2007, 301, 85–92 CrossRef CAS PubMed
.
- S. Yu, M. Ma, J. Liu, J. Tao, M. Liu and C. Gao, J. Membr. Sci., 2011, 379, 164–173 CrossRef CAS PubMed
.
- R. Nadler and S. Srebnik, J. Membr. Sci., 2008, 315, 100–105 CrossRef CAS PubMed
.
- S. S. Dhumal and A. K. Suresh, Polymer, 2009, 50, 5851–5864 CrossRef CAS PubMed
.
- W. Xie, G. M. Geise and B. D. Freeman, J. Membr. Sci., 2012, 403, 152–161 CrossRef PubMed
.
- D. Hu, Z.-L. Xu and C. Chen, Desalination, 2012, 301, 75–81 CrossRef CAS PubMed
.
- A. Ahmad and B. Ooi, J. Membr. Sci., 2005, 255, 67–77 CrossRef CAS PubMed
.
- B. Su, T. Wang, Z. Wang, X. Gao and C. Gao, J. Membr. Sci., 2012, 423–424, 324–331 CrossRef CAS PubMed
.
- A. Abidi, N. Gherraf, S. Ladjel, M. Rabiller-Baudry and T. Bouchami, Arabian J. Chem., 2011 DOI:10.1016/j.arabjc.2011.04.014
.
- P. Daraei, S. S. Madaeni, N. Ghaemi, H. Ahmadi Monfared and M. A. Khadivi, Sep. Purif. Technol., 2013, 104, 32–44 CrossRef CAS PubMed
.
- J. M. M. Peeters, J. P. Boom and M. H. V. Mulder, J. Membr. Sci., 1998, 145, 199–209 CrossRef CAS
.
- X.-X. Xu, C.-L. Zhou, B.-R. Zeng, H.-P. Xia, W.-G. Lan and X.-M. He, Sep. Purif. Technol., 2012, 96, 229–236 CrossRef CAS PubMed
.
- Y. Li, Y. Su, J. Li, X. Zhao, X. Fan, J. Zhu, Y. Ma, Y. Liu and Z. Jiang, J. Membr. Sci., 2015, 476, 10–19 CrossRef CAS PubMed
.
- B. B. Tang, Z. B. Huo and P. Y. Wu, J. Membr. Sci., 2008, 320, 198–205 CrossRef CAS PubMed
.
- H. W. Sun, G. H. Chen, R. H. Huang and C. J. Gao, J. Membr. Sci., 2007, 297, 51–58 CrossRef CAS PubMed
.
- Y.-C. Chiang, Y. Chang, C.-J. Chuang and R.-C. Ruaan, J. Membr. Sci., 2012, 389, 76–82 CrossRef CAS PubMed
.
- Y.-L. Ji, Q.-F. An, Q. Zhao, W.-D. Sun, K.-R. Lee, H.-L. Chen and C.-J. Gao, J. Membr. Sci., 2012, 390–391, 243–253 CrossRef CAS PubMed
.
- Y.-N. Wang and C. Y. Tang, J. Membr. Sci., 2011, 376, 275–282 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |