A new sensing platform based on electrospun copper oxide/ionic liquid nanocomposite for selective determination of risperidone
Abstract
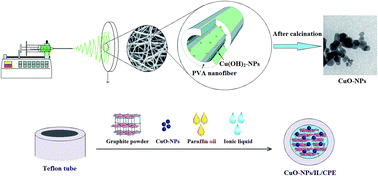
A new synthetic method for the fabrication of copper oxide nanoparticles and its applications towards the electrochemical sensing of risperidone (Ris) are reported. The synthesized nanoparticles were characterized by different methods such as transmission electron microscopy (TEM), scanning electron microscopy (SEM) and energy dispersive X-ray analysis (EDX). The copper oxide nanoparticle/ionic liquid (CuO-NPs/IL) nanocomposite modified electrode exhibits excellent electrocatalytic activity towards the oxidation of Ris compared to a carbon paste electrode (CPE) and CuO-NP/CPE. The dependence of the oxidation peak current on the pH of the solution, amount of modifier, scan rate and concentration of the analyte was studied to optimize the experimental conditions. The detection limit of 0.016 μM and two linear calibration ranges of 0.1–1 and 1–100 μM were obtained for Ris determination at CuO-NP/IL/CPE. The proposed modified electrode was successfully applied for the determination of Ris in human blood serum samples without separation or pretreatment.

 Please wait while we load your content...
Please wait while we load your content...