Fabrication of attapulgite/carbon composites from spent bleaching earth for the efficient adsorption of methylene blue
Jie Tangab,
Bin Mua,
Li Zonga,
Maosong Zhenga and
Aiqin Wang*a
aCenter of Xuyi Attapulgite Applied Technology, State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, P. R. China. E-mail: aqwang@licp.cas.cn; Fax: +86 931 8277088; Tel: +86 931 4968118
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 1st April 2015
Abstract
In this work, one-dimensional attapulgite/carbon composites were prepared by a one-step carbonization process using the residual organic matter of spent bleaching earth as a low-cost available carbon precursor. The obtained composites were characterized by transmission electron microscopy, Fourier transform infrared spectroscopy, X-ray diffraction, and thermogravimetric analysis to confirm the presence of carbonaceous species. The attapulgite/carbon composites can be used as adsorbents for the removal of methylene blue, with a maximum adsorption capacity of 132.72 mg g−1, and the process parameters affecting the adsorption behavior for methylene blue, such as the pH of the solution, calcination temperature and contact time, were also analyzed through batch adsorption processes. It was revealed that attapulgite/carbon composites could be employed as candidates for the removal of cationic dyes.
1. Introduction
Among various dyes, cationic dyes have been commonly used for different purposes due to their ease of good fastness, durability, and applicability to materials.1,2 However, it is well-known that most organic dyes are carcinogenic and mutagenic, and the discharge of organic dyes results in a serious detriment, even at very low concentrations, to the entire ecosystem and living body.3,4 To date, adsorption technology has been considered to be a front line of defence against pollutants, using adsorbents, because of its simplicity, high efficiency and low operational costs, thus it has become one of the preferable choices for the restoration, remediation and sustainability of our environment.5,6 In recent years, several efforts have been made by previous researchers to tackle dyeing industry effluents through the development and application of different adsorbents.7,8Clay mineral/carbon composites, as novel adsorbents, have recently received considerable attention for their potential applications in the removal of dyestuff from dyeing industry wastewater.9,10 Many researchers have used furfuryl alcohol,11 sucrose,12,13 or cellulose acetate14 as carbon precursors to prepare clay mineral/carbon composites to serve as adsorbents or catalysts. From a comprehensive literature review, adsorbents with high applicability and low cost in adsorption processes are the most desirable choice. However, most of the methods for the preparation of clay mineral/carbon composites reported in the previous studies are complicated, and the raw materials are expensive. Hence, the development of clay mineral/carbon composites with good adsorption capacities is urgently in demand, using low-cost available carbon precursors.
Crude palm oil usually contains many impurities, coloring matter and other hazardous substances, which are bad for health and the oil’s quality. Therefore, it is indispensable for it to be refined to remove the above components, improving the appearance and quality of the oil before eating.15 The refining process for crude palm oil usually includes three operations: degumming, bleaching and deodorization.16 The bleaching process is the most crucial step, which is realized by the use of activated clay at a dosage of 1–3%, and then the activated clay will become the spent bleaching earth (SBE) after bleaching, which contains about 10–21% normal grease. Therefore, it is a rational and feasible strategy to prepare clay mineral/carbon composites using the residual organic matter of the spent bleaching earth as a cheap carbon precursor. Hussein et al. prepared kaolin/carbon adsorbents with and without sulphuric acid pretreatment, followed by activation–carbonization at 500 °C for carotene removal from red palm oil.17 Mana et al. reported a treated SBE, by impregnation with a 1.0 N sodium hydroxide solution followed by mild thermal treatment (100 °C), for the removal of basic dyes and lead from aqueous solutions.18,19 Anadão et al. prepared carbon/montmorillonite composites from waste bleaching sodium montmorillonite for the adsorption of methylene blue (MB) and gasoline.20 In addition, clay mineral/carbon adsorbents were also fabricated by the pyrolysis of waste materials based on natural bentonite in the presence of tetrachloromethane.21 It is not hard to find that research on SBE was mainly centred around two-dimensional clay materials,20 but one-dimensional clay-based SBE has scarcely been studied, despite that natural attapulgite-based bleaching earth has received growing attention as an eco-friendly decolorizer.22 One-dimensional nanomaterials, especially the natural ones, exhibit fascinating properties based on their atomic scale structures and their 1D morphology, and they can also act as ideal carriers to fabricate various functional materials for potential magnetic, catalytic and adsorption applications.23–25 It is feasible to fabricate carbon adsorption sites on the surface of attapulgite (APT) to derive new composite materials. Therefore, attapulgite/carbon (APT/C) composites were prepared by a one-step carbonization process, using one-dimensional APT-based SBE as a low-cost available raw material, for the adsorption of MB in this study, as illustrated in Scheme 1. In addition, the adsorption mechanism and the process parameters affecting the adsorption behavior were also investigated.
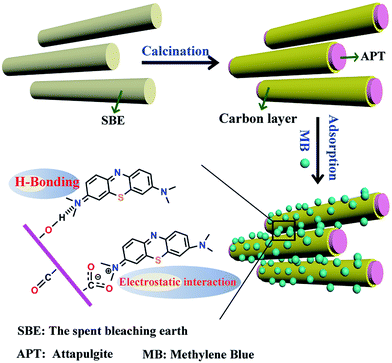 | ||
| Scheme 1 Schematic illustration of the synthetic route of the APT/C composites for the adsorption of MB. | ||
2. Materials and methods
2.1. Materials
SBE, which is mainly composed of APT and about 21% organic matter (such as grease, natural pigments, fatty acids, etc.), was provided by Jinguang Food Co. LTD (Zhejiang, China). MB (indicator grade) with a formula of C16H18N3SCl was purchased from Alfa Aesar, a Johnson Matthey Company (UK) and used as received. All of the other chemicals were analytical grade and used without further purification, and all solutions were prepared with deionized water.2.2. Preparation of the attapulgite/carbon (APT/C) composites
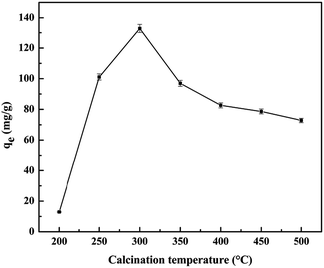
25.0 g of SBE was calcinated under temperatures of 200, 250, 300, 350, 400, 450 and 500 °C, respectively. The calcination process was carried out in an air atmosphere up to the final temperature, with a heating rate of 12 °C min−1, and keeping at the final temperature for 2 h. The samples were marked as APT/C-200, APT/C-250, APT/C-300, APT/C-350, APT/C-400, APT/C-450, and APT/C-500, corresponding to the above calcinated temperatures, respectively.2.3. Adsorption experiments
Adsorption measurements were performed in a series of 50 mL conical flasks containing 0.025 g of adsorbent and 25 mL of the MB solution. The mixtures were shaken in a thermostatic shaker (THZ-98A) at 30 °C and 160 rpm for a given time, and then the adsorbent was separated by direct filtration. The concentrations of the MB solution before and after adsorption were analyzed using a Specord 200 UV-vis spectrophotometer, by monitoring the absorbance changes at the wavelength corresponding to maximum absorbance (664 nm). The adsorption capacity of the adsorbent for MB was calculated according to the following equation:
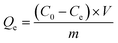 | (1) |
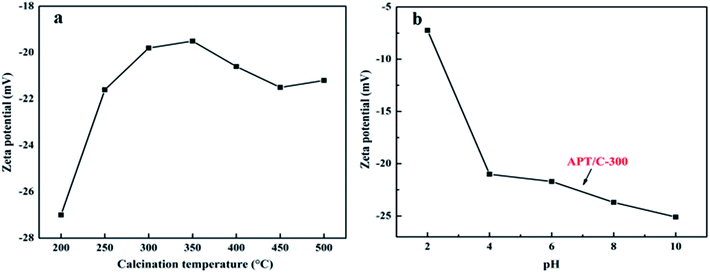
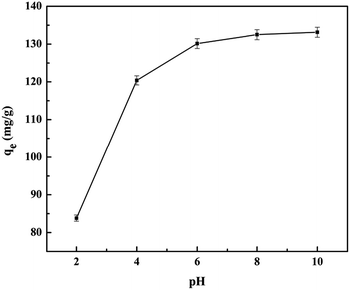
The effect of the pH on dye adsorption was investigated according to a similar procedure that kept the same initial dye concentration (200 mg L−1) while maintaining the pH of the initial solution at 2.0 and 10.0, respectively.
2.4. Reusability of the APT/C composite
In order to evaluate the regeneration ability of the APT/C composite, the desorption process and readsorption of MB were studied for five consecutive cycles. In each cycle, the APT/C composite was added to a 200 mg L−1 MB solution and the mixture was placed in a thermostatic shaker and shaken for 360 min at 160 rpm. Then the MB-adsorbed APT/C composite was separated from the MB solution by centrifugation and added to 25 mL of 1.0 mol L−1 CH3COOH and shaken for 360 min. After desorption, the APT/C composite was collected and the amount of desorbed dye was examined using a UV-vis spectrophotometer. After each cycle of the desorption and readsorption experiments, the desorbed APT/C composite was activated with NaOH (0.1 mol L−1) solution and then washed with deionized water to recondition and neutralize the adsorbent for further adsorption of MB.2.5. Characterization
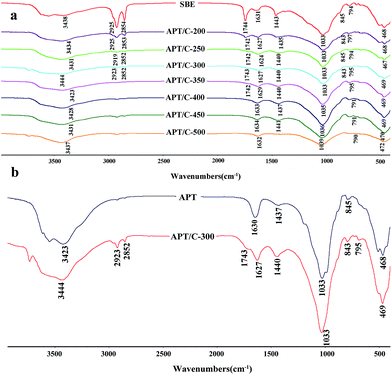
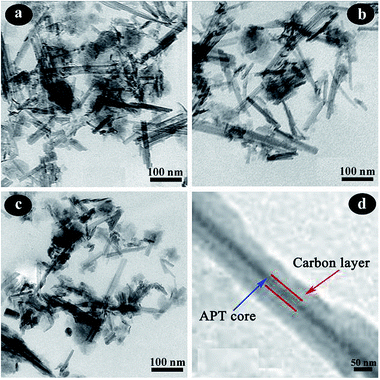
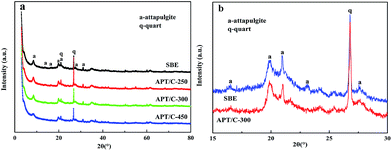
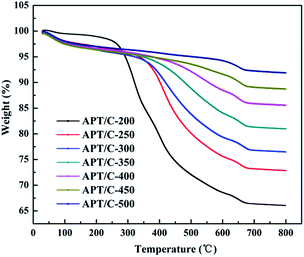
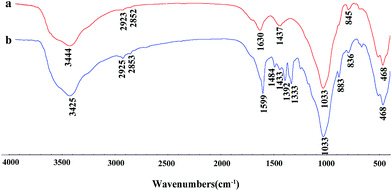
Fourier transform infrared (FTIR) spectra of the samples were recorded with a Fourier transform infrared spectrometer (Thermo Nicolet NEXUS TM, USA) in the range of 4000–400 cm−1 using KBr pellets. The morphologies of the samples were observed using a JSM-6701F field emission scanning electron microscope (JEOL, Tokyo, Japan) at an acceleration voltage of 10.0 kV and a working distance of 10 mm, in high vacuum mode, after coating the sample with a gold film. The TEM images were taken using a JEM-2010 high resolution transmission electron microscope (JEOL, Tokyo, Japan) at an acceleration voltage of 200 kV, and the sample was ultrasonically dispersed in anhydrous ethanol and dropped onto a grid before observation. Powder X-ray diffraction (XRD) (Pana XPERT PRO, Netherlands) patterns were obtained using an X-ray diffractometer with Cu Kα (1.540598 Å) radiation at a scan rate of 0.05° s−1, running at 40 kV and 30 mA. The weight percent of carbonaceous species was determined by thermogravimetric analysis (TGA) (STA 6000, Perkin Elmer, USA) at a heating rate of 10 °C min−1 from 30 °C to 800 °C in an oxygen atmosphere. The specific surface area was determined using the Brunauer–Emmett–Teller (BET) method and the pore volume was estimated using the Barrett–Joyner–Halenda (BJH) method at 77 K (ASAP 2020 M, Micromeritics Instrument Corporation, USA). The samples were dried and outgassed at 105 °C for 4 h before N2 adsorption. The zeta potentials of the suspensions were conducted using a zeta potential instrument (Malvern Zeta voltmeter (ZEN3600), Britain). UV-vis spectra were determined using a UV-vis spectrophotometer (SPECORD 200, Analytik Jera AG).3. Results and discussion
3.1. Characterization of the APT/C composites
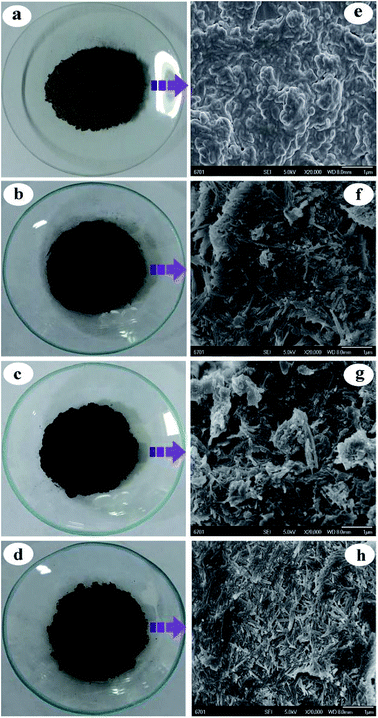 | ||
| Fig. 2 Digital photographs of (a) SBE, (b) APT/C-250, (c) APT/C-300 and (d) APT/C-450, and SEM images of (e) SBE, (f) APT/C-250, (g) APT/C-300 and (h) APT/C-450. | ||
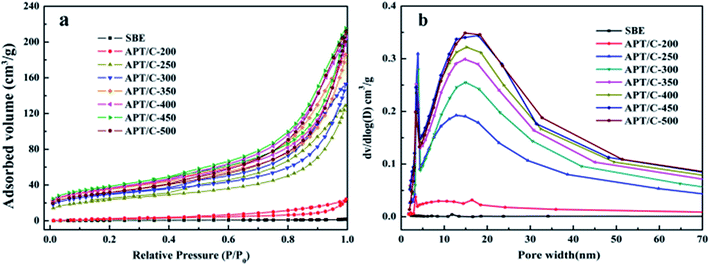 | ||
| Fig. 6 (a) N2 adsorption/desorption isotherms and (b) BJH pore size distributions of the SBE and APT/C composites. | ||
A summary of the pore structure parameters of all samples is listed in Table 1. It can be seen that the BET surface area of the APT/C composites firstly gradually increases in the range of 200–450 °C, with the increasing calcination temperature, and then slightly decreases when the temperature is above 450 °C. The increment in the specific surface area can be mainly attributed to the decomposition of the organic matter, which provides access for nitrogen molecules into some of the pores, or when the temperature is lower than 300 °C, zeolite water and a part of the bound water can be removed from the nanoporous tunnels of APT. In a word, such a change can improve the adsorption properties for MB to some extent. The remaining bound water and a small quantity of the structural water are removed when the temperature increases from 300 to 450 °C, which leads to partial collapse of the nanopores. While at the higher calcination temperature (T > 450 °C), the specific surface area slightly decreases. This phenomenon can be ascribed to the collapse of the pores and channels of APT, and the decomposition of the loaded carbon species. Therefore, the decrement in the specific surface area may result in a reduced adsorption capacity.33
| Sample | SBETa (m2 g−1) | Smicb (m2 g−1) | Sextc (m2 g−1) | Vtotd (cm3 g−1) | Vmice (cm3 g−1) | Dporef (nm) |
|---|---|---|---|---|---|---|
| a BET (Brunauer–Emmett–Teller) surface area.b Micropore surface area, derived from a t-plot method.c External surface area, calculated using a t-plot method.d Total pore volume, measured at P/P0 = 0.974.e Micropore volume, obtained from a t-plot method.f Average pore diameter, calculated from Dpore = 4 V/A according to BET. | ||||||
| SBE | 2.34 | — | 3.49 | 0.0022 | — | 3.85 |
| APT/C-200 | 12.69 | — | 18.38 | 0.0266 | — | 8.38 |
| APT/C-250 | 85.19 | 9.00 | 76.19 | 0.1699 | 0.0031 | 7.98 |
| APT/C-300 | 104.92 | 19.32 | 85.60 | 0.2011 | 0.0080 | 7.67 |
| APT/C-350 | 125.12 | 18.82 | 106.31 | 0.2477 | 0.0077 | 7.92 |
| APT/C-400 | 129.48 | 17.56 | 111.92 | 0.2719 | 0.0071 | 8.40 |
| APT/C-450 | 137.16 | 23.36 | 113.81 | 0.2859 | 0.0099 | 8.34 |
| APT/C-500 | 112.67 | 18.87 | 93.80 | 0.2749 | 0.0079 | 9.76 |
As the temperature increases, the samples have a much wider pore size distribution; therefore, the corresponding pore size distributions were investigated according to BJH theory (Fig. 6b). The larger pores in the range of 5–35 nm correspond to mesopores formed by decomposition of the organic matter in the SBE, or the removal of coordinated water and a small quantity of the structural water existing in the crystal structure of APT leading to a change of the original conformation of APT during calcination. Thus, it can be concluded that mesopores (5–35 nm) may be the main location for the interactions between the dye molecules and APT/C composites.
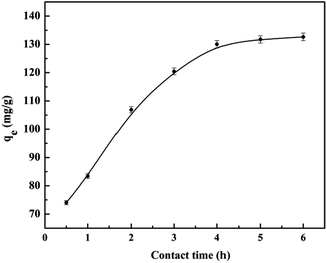
3.2. Adsorption properties
3.3. Reusability of adsorbent
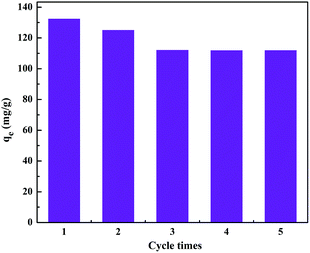
Reusability is a very important and critical factor in the design of a progressive and efficient adsorbent. A good adsorbent should have a high adsorption capacity as well as a high desorption efficiency, which will reduce the overall cost of the adsorbent.In this study, the MB-adsorbed APT/C composite (APT/C-300) was treated with 1.0 mol L−1 CH3COOH solution, as the desorbing agent, to regenerate the sorption sites, which was proven to be suitable for desorption of MB due to the similar consistency and electrostatic repulsions.43 Afterwards, the desorbed adsorbent was treated with 0.1 mol L−1 NaOH solution as the activator for MB adsorption. Then the regenerated adsorbent was utilized again to adsorb the MB solution to study its adsorption stability, and five successive cycles were performed (Fig. 12).
It was observed that the adsorption capacity of APT/C-300 is still higher than 112 mg g−1 after regeneration for five times, indicating that this composite has an excellent adsorption capacity and stability. The slight decrease of the adsorption capacity may be attributed to the incomplete desorption of the MB adsorbed on APT/C-300. Accordingly, the results from the regeneration experiments show that the as-prepared APT/C composite can be used as an efficient recyclable adsorbent for the treatment of wastewater.
4. Conclusions
In this work, APT/C composite adsorbents have been fabricated via a one-pot carbonization process using a low-cost available carbon precursor. The TEM images clearly revealed that the carbonaceous species were successfully coated on the surface of APT, which can also be well validated by the FTIR spectra and TGA. It was found that the obtained adsorbent showed a high adsorption capacity of 132.72 mg g−1 for the removal of MB, and the electrostatic attractions and hydrogen-bonding between the APT/C composites and MB molecules play a primary role in the whole adsorption process. Thus, the as-prepared adsorbent could serve as a low-cost and environmentally friendly adsorption material for the removal of dyes from water, due to the advantages of facile preparation, cheap raw materials, and no requirement for additional chemical reagents. It is also anticipated that a feasible approach to realize the application of spent bleaching earth in wastewater treatment could be developed.Acknowledgements
The authors would like to thank the “863” Project of the Ministry of Science and Technology, P. R. China (no. 2013AA032003) and the National Natural Science Foundation of China (21377135) for the financial support of this research.Notes and references
- Y. F. Tang, T. Hu, Y. D. Zeng, Q. Zhou and Y. Z. Peng, RSC Adv., 2015, 5, 3757 RSC.
- M. Auta and B. H. Hameed, Chem. Eng. J., 2014, 237, 352 CrossRef CAS PubMed.
- M. Ghaedi, A. G. Nasab, S. Khodadoust, M. Rajabi and S. Azizian, J. Ind. Eng. Chem., 2014, 20, 2317 CrossRef CAS PubMed.
- A. S. Bhatt, P. L. Sakaria, M. Vasudevan, R. R. Pawar, N. Sudheesh, H. C. Bajaj and H. M. Mody, RSC Adv., 2012, 2, 8663 RSC.
- T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Adv. Colloid Interface Sci., 2014, 209, 172 CrossRef PubMed.
- M. A. M. Salleh, D. K. Mahmoud, W. A. Karim and A. Idris, Desalination, 2011, 280, 1 CrossRef CAS PubMed.
- M. Rafatullah, O. Sulaiman, R. Hashim and A. Ahmad, J. Hazard. Mater., 2010, 177, 70 CrossRef CAS PubMed.
- V. K. Gupta and Suhas, J. Environ. Manage., 2009, 90, 2313 CrossRef CAS PubMed.
- C. H. Zhou, Appl. Clay Sci., 2011, 53, 85 CrossRef CAS PubMed.
- S. M. Koh and J. B. Dixon, Appl. Clay Sci., 2001, 18, 111 CrossRef CAS.
- P. R. Shukla, S. B. Wang, H. M. Ang and M. O. Tadé, Adv. Powder Technol., 2009, 20, 245 CrossRef CAS PubMed.
- P. Anadão, E. A. Hildebrando, I. L. R. Pajolli, K. R. O. Pereira, H. Wiebeck and F. R. V. Díaz, Appl. Clay Sci., 2011, 53, 288 CrossRef PubMed.
- A. Gómez-Avilés, M. Darder, P. Aranda and E. Ruiz-Hitzky, Appl. Clay Sci., 2010, 47, 203 CrossRef PubMed.
- C. H. Zhou, D. Zhang, D. S. Tong, L. M. Wu, W. H. Yu and S. Ismadji, Chem. Eng. J., 2012, 209, 223 CrossRef CAS PubMed.
- C. J. Vincent, R. Shamsudin and A. S. Baharuddin, J. Food Eng., 2014, 143, 123 CrossRef CAS PubMed.
- M. Mana, M. S. Ouali, L. C. de Menorval, J. J. Zajac and C. Charnay, Chem. Eng. J., 2011, 174, 275 CrossRef CAS PubMed.
- M. Z. B. Hussein, D. Kuang, Z. Zainal and T. K. Teck, J. Colloid Interface Sci., 2001, 235, 93 CrossRef PubMed.
- M. Mana, M. S. Ouali and L. C. de Menorval, J. Colloid Interface Sci., 2007, 307, 9 CrossRef CAS PubMed.
- M. Mana, M. S. Oualia, M. Lindheimer and L. C. de Menorval, J. Hazard. Mater., 2008, 159, 358 CrossRef CAS PubMed.
- P. Anadão, I. L. R. Pajolli, E. A. Hildebrando and H. Wiebeck, Adv. Powder Technol., 2014, 25, 926 CrossRef PubMed.
- R. Leboda, B. Charmas, J. Skubiszewska-Zięba, S. Chodorowski, P. Oleszczuk, V. M. Gun’ko and V. A. Pokrovskiy, J. Colloid Interface Sci., 2005, 284, 39 CrossRef CAS PubMed.
- G. Y. Tian, W. B. Wang, B. Mu, Y. R. Kang and A. Q. Wang, J. Taiwan Inst. Chem. Eng., 2015 DOI:10.1016/j.jtice.2014.12.021.
- A. B. Zhang, S. T. Liu, K. K. Yan, Y. Ye and X. G. Chen, RSC Adv., 2014, 4, 13565 RSC.
- J. D. Dai, X. Wei, Z. J. Cao, Z. P. Zhou, P. Yu, J. M. Pan, T. B. Zou, C. X. Li and Y. S. Yan, RSC Adv., 2014, 4, 7967 RSC.
- W. B. Wang, F. F. Wang, Y. R. Kang and A. Q. Wang, RSC Adv., 2013, 3, 11515 RSC.
- M. Ghaedi, M. D. Ghazanfarkhani, S. Khodadoust, N. Sohrabi and M. Oftade, J. Ind. Eng. Chem., 2014, 20, 2548 CrossRef CAS PubMed.
- S. Kadi, S. Lellou, K. Marouf-Khelifa, J. Schott, I. Gener-Batonneau and A. Khelifa, Microporous Mesoporous Mater., 2012, 158, 47 CrossRef CAS PubMed.
- L. F. Chen, H. W. Liang, Y. Lu, C. H. Cui and S. H. Yu, Langmuir, 2011, 27, 8998 CrossRef CAS PubMed.
- P. Anadão, I. L. R. Pajolli, E. A. Hildebrando and H. Wiebeck, Adv. Powder Technol., 2014, 25, 926 CrossRef PubMed.
- J. Madejová, Vib. Spectrosc., 2003, 31, 1 CrossRef.
- J. M. Hong, B. Lin, J. S. Jiang, B. Y. Chen and C. T. Chang, J. Ind. Eng. Chem., 2014, 20, 3667 CrossRef CAS PubMed.
- Y. Liu, Y. R. Kang, B. Mu and A. Q. Wang, Chem. Eng. J., 2014, 237, 403 CrossRef CAS PubMed.
- H. Chen, J. Zhao, A. G. Zhong and Y. X. Jin, Chem. Eng. J., 2011, 174, 143 CrossRef CAS PubMed.
- C. H. Wang, M. L. Auad, N. E. Marcovich and S. Nutt, J. Appl. Polym. Sci., 2008, 109, 2562 CrossRef CAS PubMed.
- R. L. Frost and Z. Ding, Thermochim. Acta, 2003, 397, 119 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- J. P. Zhang, Q. Wang, H. Chen and A. Q. Wang, Clay Miner., 2010, 45, 145 CrossRef CAS PubMed.
- A. A. Nunes, A. S. Franca and L. S. Oliveira, Bioresour. Technol., 2009, 100, 1786 CrossRef CAS PubMed.
- M. M. Hamed, I. M. Ahmed and S. S. Metwally, J. Ind. Eng. Chem., 2014, 20, 2370 CrossRef CAS PubMed.
- S. Cengiz and L. Cavas, Bioresour. Technol., 2008, 99, 2357 CrossRef CAS PubMed.
- Z. Sun, C. J. Li and D. Y. Wu, J. Chem. Technol. Biotechnol., 2010, 85, 845 CrossRef CAS PubMed.
- A. A. Futaisi, A. Jamrah and R. A. Hanai, Desalination, 2007, 214, 327 CrossRef PubMed.
- Q. Zhou, Q. Gao, W. J. Luo, C. J. Yan, Z. N. Ji and P. Duan, Colloids Surf., A, 2015, 470, 248 CrossRef CAS PubMed.
- A. K. Chowdhury, A. D. Sarkar and A. Bandyopadhyay, Clean: Soil, Air, Water, 2009, 37, 581 CrossRef CAS PubMed.
- M. Auta and B. H. Hameed, Chem. Eng. J., 2012, 198–199, 219 CrossRef CAS PubMed.
- L. Liu, Y. R. Zhang, Y. J. He, Y. F. Xie, L. H. Huang, S. Z. Tan and X. Cai, RSC Adv., 2015, 5, 3965 RSC.
| This journal is © The Royal Society of Chemistry 2015 |