Enhanced catalytic performance of Pd–Pt nanodendrites for ligand-free Suzuki cross-coupling reactions†
Abstract
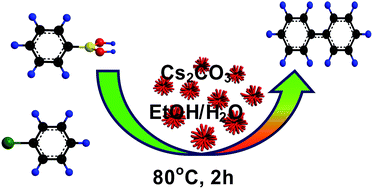
Pd–Pt nanodendrites were synthesized by a facile, environment-friendly, one-pot wet-chemical method, without using any seed, template, or toxic organic solvent. The as-obtained bimetallic nanocrystals exhibited efficient catalytic activity toward ligand-free Suzuki cross-coupling reaction in ethanol aqueous solution. A series of biphenyl compounds were obtained with high yields under mild conditions. Furthermore, the catalyst could be easily recovered and reused at least 6 consecutive cycles, nearly without losing its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...