Ga-based magnetic fluid with Al2O3-coated Ni nanoparticles
Shuchun Zhao,
Chuncheng Yang,
Xiufang Bian*,
Tongxiao Guo and
Mengchun Yu
Key Laboratory for Liquid-Solid Structural Evolution and Processing of Materials, Ministry of Education, Shandong University, Jinan 250061, China. E-mail: xfbian@sdu.edu.cn; Fax: +86 0531 88395011; Tel: +86 0531 88392748
First published on 29th April 2015
Abstract
Five kinds of metal-based magnetic fluids were prepared by dispersing different kinds of Ni nanoparticles (NPs) into liquid Ga: (a) neat Ni NPs, (b) Al2O3-coated Ni NPs, (c) SiO2-coated Ni NPs, (d) Ni NPs after 573 K heat treatment, (e) Ni NPs after 673 K heat treatment. The stabilities and magnetic properties of the five different magnetic fluids were characterized. The saturation magnetizations of the magnetic fluids with Al2O3-coated and SiO2-coated Ni NPs are 1.56 emu g−1 and 1.36 emu g−1, respectively, higher than 0.81 emu g−1 and 0 emu g−1, which are the saturation magnetizations of magnetic fluids with Ni NPs after 573 K and 673 K heat treatment respectively. Furthermore, the results of magnetic weight experiments show that the magnetic fluid with Al2O3-coated Ni NPs and magnetic fluid with Ni NPs after 573 K heat treatment have less magnetic loss. It can be concluded that Ga-based magnetic fluid with Al2O3-coated Ni NPs has both high magnetic properties and strong stability, which is suitable for practical applications. Compared with the 673 K heat treatment, the 573 K heat treatment of Ni NPs also enhances the stability of the magnetic fluid with relatively less magnetic property loss.
1. Introduction
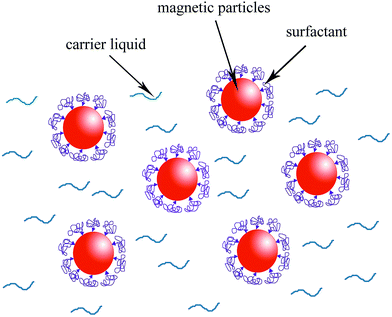
Magnetic fluids, as function materials, have received much attention in recent years due to their normal liquid behaviors coupled with magnetic properties.1–4 As they exhibit unique properties, magnetic fluids offer applications in many fields, such as sealing,5 lubricating,6 removal of water pollutants,7 target drug delivery,8 printed electronics,9 and so on. Stability is a significant factor directly affecting the practical applications of magnetic fluids. Generally speaking, magnetic NPs tend to sedimentate and aggregate due to strong magnetic dipole–dipole attraction between particles, which could lead to the poor stability of the magnetic fluid.10 Typical method to prevent the sedimentation and aggregation among the NPs is to modify the surfaces of magnetic particles.11,12 In terms of traditional magnetic fluid, such as organics or water-based magnetic fluid,13–15 researches about the stability have achieved encouraging progress. Vaidyanathan16 adopted oleic acid as a monolayer surfactant to modify the Co1−xZnxFe2O4 NPs. Zhang17 stabilized the magnetic fluid by using polymeric steric stabilization layer as a bilayer surfactant. The basic structure of magnetic fluid is shown in Fig. 1. The surfactant plays a role in two aspects: on the one hand, it can prevent agglomeration among magnetic particles; on the other hand, the high affinity between surfactant and carrier liquid ensures that the sedimentation of magnetic particles does not occur.Metal-based magnetic fluid, compared with traditional magnetic fluid, presents superior performance in some ways, such as higher melting point, superior thermal and electrical conductivity.18,19 All the merits make metal-based magnetic fluid a good choice for the application in magneto-caloric energy conversion and heat exchange devices, where a high thermomagnetic coefficient is required.20 However, reports on this kind of magnetic fluid are scarce, with only a study of metal-based magnetic functional fluids with amorphous particles21 and a study of fabrication of magnetic fluid through loading Ni particles into gallium.22 More unfortunately, published literatures about improving the stability of metal-based magnetic fluid are extremely rare.
In this paper, we present a feasible way to enhance the stability of magnetic Ni NPs dispersed uniformly in the liquid Ga. Al2O3 is used for the first time as a coating layer to improve the stability of Ga-based magnetic fluid with Ni NPs. And heat treatment is firstly introduced as a method to improve the stability of the magnetic fluid. Interpretations for the fundamental mechanism are given reasonably and are expected to guide the practical applications of Ga-based magnetic fluid.
2. Experimental
All the chemicals used in the experiments were of analytical reagent (AR) grade without further purification.2.1 Coating Ni NPs with Al2O3
The Al2O3 layer was obtained by a chemical precipitation method. The process was carried out by two steps with the following reactions, respectively:| Al(NO3)3 + 3NH3·H2O → Al(OH)3↓ + 3NH4NO3 | (1) |
| 2Al(OH)3 → Al2O3 + 3H2O | (2) |
In the first reaction eqn (1), Al(OH)3 layer was synthesized in a suspension. Certain amounts of PEG-2000 and PEG-4000 were dissolved as dispersants and Ni NPs were added as nucleation centers, and then the PH of the suspension was adjusted to 9. After mechanical stirring the suspension for 5 hours, The Al(OH)3-coated Ni NPs were obtained by washing, drying and grinding the precipitate.
The second reaction eqn (2) was a process of Al(OH)3 layer transforming into Al2O3 layer which occurred in a mixture of Al(OH)3-coated Ni NPs and MgSO4 powders (the atomic ratio of Mg/Al was adjusted to 20) at a specific high temperature. MgSO4 powders were added as an isolation phase to avoid the agglomeration of Al2O3-coated Ni NPs. The mixture was prepared by adding the Al(OH)3-coated Ni NPs into the pre-made MgSO4 solution with ultrasonic dispersion to get the suspension and mechanical stirring the suspension at 353 K until the water was dried. Finally, the Al2O3-coated Ni NPs were obtained by washing the transformed mixture four times with distilled water to remove the MgSO4 powders.
2.2 Coating Ni NPs with SiO2
Synthesis of the SiO2 layer was carried out by a typical sol–gel process.23 TEOS was chosen as the precursor and CH3COONH4 as the catalyst. The chemical reaction was as follows:| Si(OC2H5)4 + 2H2O → SiO2 + 4C2H5OH | (3) |
The reaction occurred in an alcohol/water (in volume ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture containing Ni NPs as nucleation centers. The PH of the suspension was adjusted to 10. The reaction system was placed in a thermostat water bath to keep the temperature at 323 K. After stirring for 60 min, the obtained particles were washed with distilled water for 4 times, and then dried in a desiccator (temperature kept at 353 K). Finally, the SiO2-coated Ni NPs were obtained.
1) mixture containing Ni NPs as nucleation centers. The PH of the suspension was adjusted to 10. The reaction system was placed in a thermostat water bath to keep the temperature at 323 K. After stirring for 60 min, the obtained particles were washed with distilled water for 4 times, and then dried in a desiccator (temperature kept at 353 K). Finally, the SiO2-coated Ni NPs were obtained.
2.3 Preparation of Ga-based magnetic fluids
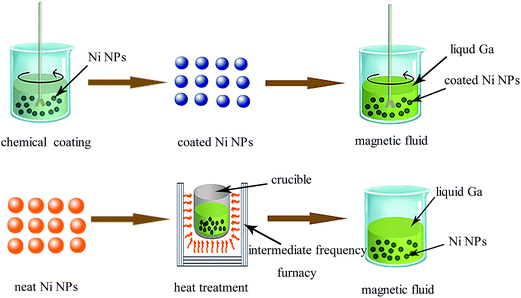
Five kinds of Ga-based magnetic fluids were made: (a) dispersing SiO2-coated Ni NPs into liquid Ga with stirring constantly; (b) dispersing Al2O3-coated Ni NPs into liquid Ga with stirring constantly; (c) heat treatment of neat Ni NPs dispersed into liquid Ga at 573 K; (d) heat treatment of neat Ni NPs dispersed into liquid Ga at 673 K; (e) dispersing neat Ni NPs into liquid Ga with stirring constantly as a blank sample. The percentage of Ni NPs added into each sample was 3.5 wt%. The schematic drawing of the preparation processes of Ga-based magnetic fluids is shown in Fig. 2.2.4 Characterization
The crystalline phase of the magnetic NPs was identified by using a commercial Rigaku Dmax-rc X-ray diffraction (XRD) equipped with a Ni-filtered Cu-Kα radiation source. The morphology and size of the magnetic NPs before and after coating process was analyzed by Transmission electron microscopy (TEM). The elemental composition of the magnetic NPs was identified by Energy dispersive X-ray spectroscopy (EDS). The magnetic hysteretic curves of the magnetic NPs and the magnetic fluids were measured by Vibrating sample magnetometer (VSM). The magnetic weight was measured by Gouy magnetic balance (GMB) and the applied magnetic field was set to 100 mT.3. Results and discussion
3.1 XRD patterns
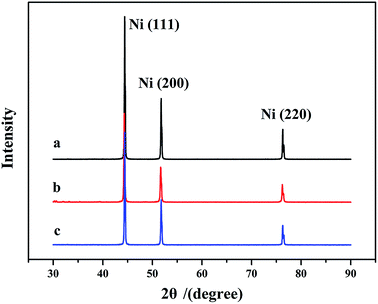
The XRD patterns of (a) neat Ni NPs, (b) SiO2-coated Ni NPs and (c) Al2O3-coated Ni NPs are illustrated in Fig. 3. A series of characteristic peaks can be seen for (111), (200) and (220) planes of neat Ni NPs, and the samples can be perfectly indexed to the face-centred cubic structure (JCPDS no. 04-0850). When the Ni NPs were coated with SiO2 and Al2O3, the same features can be observed. It indicated that the crystal structures of Ni NPs are not changed by the coating process. The average grain sizes of neat Ni NPs, SiO2-coated Ni NPs and Al2O3-coated Ni NPs are 40.05 nm, 43.41 nm and 43.25 nm, respectively, which are calculated from Scherrer equation: D = Kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where D is the average grain size, K is a constant equal to 0.94, λ is the X-ray wavelength, β is the width at half maximum of the (111) peak and θ is the diffraction angle.
θ), where D is the average grain size, K is a constant equal to 0.94, λ is the X-ray wavelength, β is the width at half maximum of the (111) peak and θ is the diffraction angle.
3.2 TEM images and EDS spectra
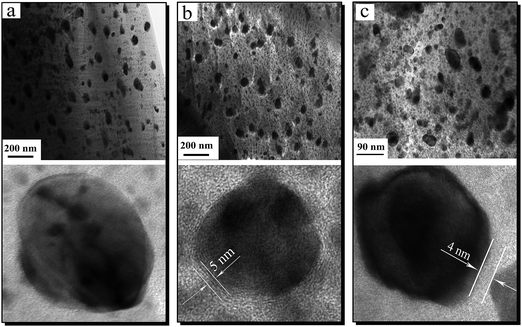
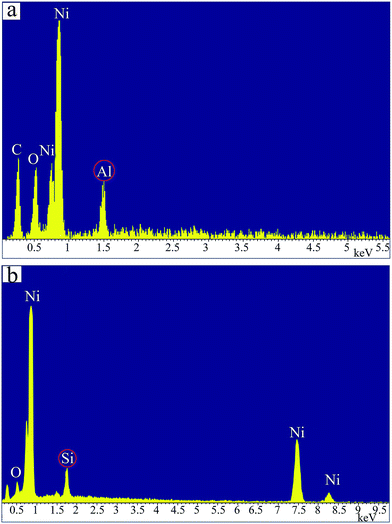
TEM images of Ni NPs before and after the coating process are shown in Fig. 4. It can be observed that the Al2O3-coated (Fig. 4(b)) and SiO2-coated (Fig. 4(c)) Ni NPs have as good dispersion as neat Ni NPs (Fig. 4(a)), indicating that the coating process did not result in conglomeration of the Ni NPs. The sizes of the coated Ni NPs range from 30 to 50 nm, which are in accordance with the result of the XRD. The high resolution TEM (HRTEM) images of respective Ni NPs indicate that the Ni NPs after coating process have visible morphology of core–shell structure. The SiO2-coated and Al2O3-coated Ni NPs have the almost same thickness, with Al2O3 shell of about 4 nm and SiO2 shell of about 5 nm respectively. In addition, the EDS spectra (Fig. 5) also show that the Al2O3 and SiO2 have been coated on the Ni NPs.3.3 Magnetic properties of Ni NPs
The magnetic properties of Ni NPs have been characterized by VSM at room temperature. The magnetic hysteresis curves of (a) neat Ni NPs, (b) Al2O3-coated Ni NPs and (c) SiO2-coated Ni NPs are shown in Fig. 6. Zero remanence and zero coercivity indicate that Al2O3-coated and SiO2-coated Ni NPs are still super-paramagnetic as the neat Ni NPs are, which means that the coating process does not change their magnetic properties. The saturation magnetization of the Ni NPs is obtained by averaging the absolute values of the maximum and minimum, and the calculated saturation magnetization of the neat Ni NPs is 50 emu g−1, which is little lower than that of bulk Ni (55 emu g−1).24 This is because the so-called size effect that the saturation magnetization of Ni nanocrystalline changes with the decrease of particle size, owing to the phenomenon that the cohesive energy of nanomaterial is lower than that of coarse-grain.25 The saturation magnetizations of Al2O3-coated and SiO2-coated Ni NPs are 44.6 emu g−1 and 38.7 emu g−1, respectively, lower than that of neat Ni NPs. It can be explained from the following two aspects: firstly, the coating layers decrease the percentage of the magnetic Ni NPs; secondly, all substances have diamagnetism to a certain degree, and the layers coated on the Ni NPs decrease the saturation magnetization by the diamagnetic contribution.26 In addition, the saturation magnetizations of Al2O3-coated and SiO2-coated Ni NPs are different, which might be caused by the different abilities of diamagnetisms between the two kinds of layers.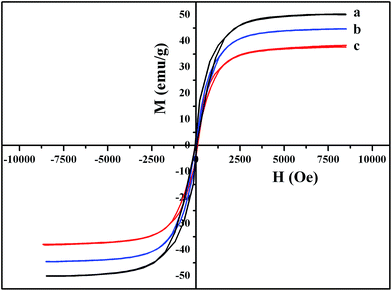 | ||
| Fig. 6 Magnetic hysteresis curves of (a) neat Ni NPs, (b) Al2O3-coated Ni NPs and (c) SiO2-coated Ni NPs. | ||
3.4 Magnetic properties of Ga-based magnetic fluid
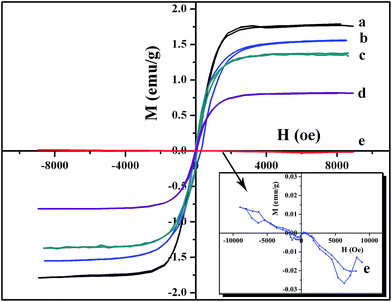
Magnetic hysteresis curves of the five different kinds of Ga-based magnetic fluids are shown in Fig. 7. The saturation magnetization of Ga-based magnetic fluid with neat Ni NPs shown in Fig. 7(a) is 1.77 emu g−1, while the saturation magnetizations of magnetic fluids after different kinds of surface treatments of Ni NPs decrease to different extent. The saturation magnetization of Ga-based magnetic fluid with Al2O3-coated Ni NPs is 1.56 emu g−1, higher than that of the magnetic fluid with SiO2-coated Ni NPs which is 1.36 emu g−1. Besides, it can be learned from Fig. 7 that the saturation magnetization of Ga-based magnetic fluid with Ni NPs after 573 K heat treatment is 0.81 emu g−1, while the saturation magnetization of Ga-based magnetic fluid with Ni NPs after 673 K heat treatment is 0 emu g−1. In fact, the sample after 673 K heat treatment exhibits a curve in opposite direction, as shown in Fig. 7(e). It can be explained that the high heat treatment temperature disappear the super-paramagnetism of Ni NPs, and as a result, the Ga-based magnetic fluid with Ni NPs after 673 K heat treatment exhibits slight diamagnetic.3.5 Stability of Ga-based magnetic fluid
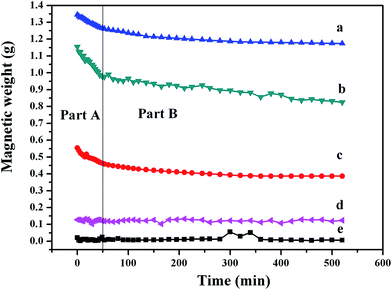
Magnetic fluid is usually used in a magnetic field, and stability is the key point of practical applications. In this part, GMB is used to evaluate the stability of Ga-based magnetic fluid. The magnetic weights of different Ga-based magnetic fluids at different time in certain magnetic field (H = 100 mT) are recorded. As shown in Fig. 8, the magnetic weight curves can be divided into two parts, the magnetic weights of all samples decrease to varying degrees in part A, while keep being stable in part B. Fig. 8(d) shows the magnetic weight curve of Ga-based magnetic fluid with neat Ni NPs, the low magnetic weight (0.12 g) indicates the poor stability of the magnetic fluid, and then demonstrates the necessary for surface treatment of Ni NPs. Ga-based magnetic fluid with Al2O3-coated Ni NPs exhibits better comprehensive properties with higher magnetic weight and stronger stability, which indicates that Al2O3 is a good choice for the coating layer. As to Ga-based magnetic fluid with SiO2-coated Ni NPs, it shows roughly the same magnetic weight as Al2O3-coated Ni NPs at the beginning, but it has a sharp reduction in part A and still declines in part B. It can be concluded that Al2O3 layer has more obvious effect on improving the stability of magnetic fluid than SiO2 layer. This can be explained from the following two aspects: on the one hand, Al2O3 and Ni can form an interface in Ni–O bond which has been demonstrated by Zhang27 using First-principles calculation method. That's to say, there is a high bonding strength between Ni and Al2O3 which can prevent the detachment between Al2O3 layer and Ni NPs; on the other hand, there is a direct relationship between the wettability and stability, and a good wettability brings out strong stability. The wettability of the solid surface is determined by chemical composition and microstructure, Nakajima28 found that solid particles with same roughness do not necessarily have the same surface contact angle, owning to the important influence of the microstructure of solid surface. It can be inferred that the special surface performance of Al2O3 make it a better coating layer than SiO2.Besides, heat treatment at different temperatures is adopted to improve the stability. The magnetic weight curve of Ga-based magnetic fluid with Ni NPs after 573 K heat treatment is shown in Fig. 8(c). It can be seen that although the curve shows a low magnetic weigh (0.55 g), it displays good stability, especially in part B. Ni NPs, because of their nanoscale dimensions, have large specific surface energy and this results in the reaction between Ni NPs and liquid Ga at lower temperatures. The reaction leads to an interfacial transition layer, which enhances the wettability between the interfaces, and thus, improves the stability. As shown in Fig. 8(e), the magnetic weight of Ga-based magnetic fluid with Ni NPs after 673 K heat treatment is 0.01 g, which is far smaller than that of the sample after 573 K heat treatment. Different surface treatments of Ni NPs lead to different magnetic weights and stability. Fig. 9 depicts the schematic presentations of surface treatments of Ni NPs. The coating process is shown in Fig. 9(a). Based on the surface of Ni NPs, the Al2O3 layer and SiO2 layer grow outward, which could not reduce the amounts of Ni. However, the heat treatment of Ni NPs (Fig. 9(b)) is a process of complex physical and chemical reaction. Based on the surface of Ni NPs, the transition layer grows inward, which could decrease the amounts of Ni. The higher the heat treatment temperature is, the more the Ni decreases. Thus, it can be inferred that the Ni NPs in the Ga-based magnetic fluid after 673 K heat treatment has reacted completely and as a result, the magnetic weight is just 0.01 g.
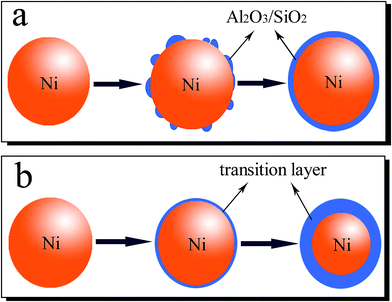 | ||
| Fig. 9 Schematic presentations of surface treatments of Ni NPs: (a) coating process, (b) heat treatment. | ||
4. Conclusions
Ga-based magnetic fluids with five different kinds of Ni NPs have been successfully prepared: (a) neat Ni NPs, (b) Al2O3-coated Ni NPs, (c) SiO2-coated Ni NPs, (d) Ni NPs after 573 K heat treatment, (e) Ni NPs after 673 K heat treatment. Results indicate that the SiO2 layer and Al2O3 layer have been coated on Ni NPs successfully and the size ranges from 30 nm to 50 nm. The saturation magnetizations of Al2O3-coated and SiO2-coated Ni NPs are 44.6 emu g−1 and 38.7 emu g−1 respectively, and the saturation magnetizations corresponding to the magnetic fluids are 1.56 emu g−1 and 1.36 emu g−1 respectively. The saturation magnetization of magnetic fluid with Ni NPs after 573 K heat treatment is 0.81 emu g−1, while Ni NPs after 673 K is 0 emu g−1. The results of GMB experiments indicate that Ga-based magnetic fluid with Al2O3-coated Ni NPs has strong stability, and Ni NPs after 573 K heat treatment has high stability, too. It can be confirmed that Al2O3-coated Ni NPs has both high magnetic properties and strong stability, which is suitable for practical applications. Besides, compared with the 673 K heat treatment, the 573 K heat treatment of Ni NPs also enhances the stability of the magnetic fluid with relatively less magnetic properties loss. More effort will be done to improve heat treatment process to enhance the stability of magnetic fluid with high magnetic properties.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundational of China (Grant no. 51371107) and Scientific and Technological Project of Shan-dong Province (Grant no. 2013GGX1027).References
- B. B. Yellen and G. Friedman, US Pat., 8678640 B2, 2014.
- B. I. Kharisov, H. V. Rasika and O. V. Kharissova, et al., RSC Adv., 2014, 4, 45354 RSC.
- D. Serantes, K. Simeonidis and M. Angelakeris, et al., J. Phys. Chem. C, 2014, 118, 5927 CAS.
- C. C. Yang, X. F. Bian and J. Y. Qin, et al., J. Mol. Liq., 2014, 196, 357 CrossRef CAS PubMed.
- Y. Mitamura, S. Arioka and D. Sakota, J. Phys.: Condens. Matter, 2008, 20, 204145 CrossRef CAS PubMed.
- M. Goldowsky, IEEE Trans. Magn., 1980, 16, 382 CrossRef.
- N. Ferroudj, J. Nzimoto and A. Davidson, et al., Appl. Catal., B, 2013, 9, 136 Search PubMed.
- J. K. Oh and J. M. Park, Prog. Polym. Sci., 2011, 36, 168 CrossRef CAS PubMed.
- Y. Zheng, Z. Z. He and J. Yang, et al., Sci. Rep., 2014, 4, 4588 Search PubMed.
- S. Genc and B. Derin, Curr. Opin. Chem. Eng., 2014, 3, 118 CrossRef PubMed.
- Y. X. Zhao, L. Zhuang and H. Shen, et al., J. Magn. Magn. Mater., 2009, 321, 377 CrossRef CAS PubMed.
- K. S. Wilson, J. D. Goff and J. S. Rifle, et al., Polym. Adv. Technol., 2005, 16, 200 CrossRef CAS PubMed.
- M. Zeltner and R. Grass, et al., J. Mater. Chem., 2012, 22, 12064 RSC.
- W. Brullot and N. K. Reddy, et al., J. Magn. Magn. Mater., 2012, 324, 1919 CrossRef CAS PubMed.
- S. García-Jimeno and J. Estelrich, Colloids Surf., A, 2013, 420, 74 CrossRef PubMed.
- G. Vaidyanathan and S. Sendhilnathan, J. Magn. Magn. Mater., 2008, 320, 803 CrossRef CAS PubMed.
- N. Jain and X. Zhang, et al., ACS Appl. Mater. Interfaces, 2011, 3, 662 CAS.
- Y. H. Tian and G. H. Su, et al., Prog. Nucl. Energy, 2013, 68, 177 CrossRef CAS PubMed.
- V. Y. Prokhorenko, et al., High Temp., 2000, 38(6), 954 CrossRef CAS.
- R. N. Singh, J. Mater. Eng. Perform., 2006, 15, 422 CrossRef CAS.
- C. C. Yang, X. F. Bian and J. Y. Qin, et al., RSC Adv., 2014, 4, 59541 RSC.
- M. Xiong, Y. Gao and J. Liu, J. Magn. Magn. Mater., 2014, 354, 279 CrossRef CAS PubMed.
- G. Dodbiba and K. Ono, et al., Int. J. Mod. Phys. B, 2011, 25, 947 CrossRef CAS.
- A. Wu, X. Yang and H. Yang, J. Alloys Compd., 2012, 513, 193 CrossRef CAS PubMed.
- W. H. Zhong and C. Q. Sun, et al., Acta Mater., 2005, 53(11), 3207 CrossRef CAS PubMed.
- H. J. Chen and Y. M. Wang, et al., Appl. Surf. Sci., 2011, 257, 10802 CrossRef CAS PubMed.
- W. Zhang and J. R. Smith, et al., Acta Mater., 2002, 50(15), 3803 CrossRef CAS.
- M. Miwa, A. Nakajima and A. Fujishima, et al., Langmuir, 2000, 16(13), 5754 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |