Dissociative ionization and Coulomb explosion of ethyl bromide under a near-infrared intense femtosecond laser field
Abstract
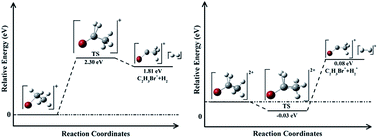
The multi-photon dissociation and Coulomb explosion of ethyl bromide C2H5Br under near-infrared (800 nm) femtosecond laser field are experimentally investigated by a DC-sliced ion imaging technique. The sliced images of fragment ions C2H5+, Br+, CH3+, CH2Br+, H2+ and C2H3Br+ are obtained, and their dissociative pathways are assigned by observing their corresponding kinetic energy release (KER) and angular distribution. It is shown that low-KER components of these fragment ions result from multi-photon dissociation of singly charged parent ion C2H5Br+, while high-KER components come from Coulomb explosion of doubly charged parent ion C2H5Br2+. It is also shown that the precursor species [C2H5+…Br+] has a longer lifetime than [C2H3Br+…H2+] and [CH3…CH2Br+]. In addition, the probable H2 and H2+ elimination channels are theoretically simulated by Gaussian 09 software packages, and the results show that the former is an asynchronous process while the latter is a synchronous process.

 Please wait while we load your content...
Please wait while we load your content...