Reduction degree and property study of graphene nanosheets prepared with different reducing agents and their applicability as a carrier of the Ru(phen)3Cl2 luminescent sensor for DNA detection†
Hongjuan
Li
a,
Jia
Wen
a,
Ruijin
Yu
a,
Caihui
Bai
a,
Yongqian
Xu
a,
Zong-Huai
Liu
b and
Shiguo
Sun
*a
aCollege of Science, Northwest A&F University, Yangling, Shaanxi 712100, PR China
bKey Laboratory of Applied Surface and Colloid Chemistry, Shaanxi Normal University, Ministry of Education, Xi'an, 710062, PR China. E-mail: sunsg@nwsuaf.edu.cn; Fax: +86-29-87082832; Tel: +86-29-87092226
First published on 2nd March 2015
Abstract
Recently, graphene nanosheets (GNS) have been widely investigated and used in capacitors, catalysts, biological/chemical sensors, etc. However, the feasible applications of GNS prepared with different reducing agents as a carrier of luminescent sensors have never been systematically studied yet. Herein, a series of GNS were acquired using different reducing agents, such as hydrazine, glucose and urea. The reduction degrees and properties of the GNS samples were systematically studied by using an X-ray diffractometer, Raman spectra, IR spectra and X-ray photoelectron spectroscopy. The results indicated that the reduction degree was in the order of hydrazine > glucose > urea, demonstrating that reducing agents play an important role in the bulk fabrication of high quality graphene. Then the GNS samples were all employed as a carrier of the Ru(phen)3Cl2 (tris(1,10-phenanthroline)ruthenium(II) dichloride) sensor to discriminate DNA. It is found that all the GNS samples can effectively quench the emission of the Ru(phen)3Cl2 sensor. After the addition of a certain amount of DNA into the corresponding systems, the luminescence intensity was fully recovered. In comparison, the luminescence response of GNS-G prepared with glucose shows the best linear correlation to the DNA added, with a detection limit of 3.62 × 10−9 g mL−1, indicating GNS-G can be employed as a good carrier of Ru(phen)3Cl2 to discriminate DNA. This work will significantly advance the research of bulk fabrication of high quality graphene and the specific applications in luminescent sensors of graphene-based functional materials in the future.
Introduction
In recent years, graphene nanosheets (GNS) have attracted wide attention due to their large specific surface area, good biocompatibility, unique electrical/thermal characteristics, etc.1–5 Owing to their unique nanostructure and excellent properties, GNS have shown potential applications in the field of capacitors, catalysts, biological/chemical sensors, cellular imaging, drug delivery, and so on.6–10At present, chemical reduction of exfoliated graphite oxide (GO) is considered as an efficient approach to produce GNS due to its low cost, economic feasibility and massive scalability.11–13 And the chemical reduction was usually carried out using hydrazine, glucose and urea as the reducing agent.14–16 However, to our knowledge, the feasible applications of GNS prepared with different reducing agents as a carrier of luminescent sensors have never been systematically studied yet.
Reduced GNS is composed of sp2 hybridized carbon atoms arranged in a honeycomb lattice with different types of oxygen-containing functional groups: carbonyl, carboxylate and epoxy groups on the basal planes.17 Because GNS is negatively charged, it can immobilize positive charged materials through both electrostatic and π–π stacking interaction.18 Previous research has shown that carbon materials can be served as effective fluorescent sensing platforms for nucleic acid detection.19–23 GNS can effectively quench the luminescence of dye through π–π stacking and electrostatic interaction.24,25 For instance, Ru(phen)3Cl2 (tris(1,10-phenanthroline)ruthenium(II) dichloride) is one type of good candidate due to the co-existing of the positive charged Ru atom and the aromatic rings of phen (the structure is shown in ESI Fig. S1†). Especially, Ru(phen)3Cl2 is the suitable candidate for deoxyribonucleic acid (DNA) site-specific, it can bind DNA by partial interaction and shows a strong preference for poly[d(A–T)] to poly[d(C–G)].26–28 After being encountered with DNA, Ru(phen)3Cl2 can be released from GNS and interacted with CT DNA immediately, leading to a luminescence recovery of Ru(phen)3Cl2. Based on this, a GO–Ru(phen)3Cl2 fluorescence material exhibiting enhanced properties was developed to image both fixed cells and live cells.29
Inspired by this, herein, a serial of GNS were fabricated using different reducing agents, such as hydrazine, glucose and urea. The reduction degrees and properties of the obtained GNS samples were systematically investigated by XRD, Raman spectra, IR spectra and XPS analysis. Then the feasible applications of GNS prepared with different reducing agents as a carrier of luminescent sensor have been systematically studied. It is found that all the GNS samples can effectively quench the emission of the Ru(phen)3Cl2 sensor. After addition of a certain amount of DNA into the corresponding systems, the luminescence intensity was all fully recovered. By comparison, the luminescence response of GNS-G prepared with glucose shows the best linear correlation to the DNA added, indicating GNS-G can be employed as a good carrier of Ru(phen)3Cl2 to discriminate DNA.
Experimental
Materials
All the chemicals are of analytical grade and were used without further purification. Natural flake graphite (325 mesh) was purchased from Alfa-Aesar Co. Calf thymus DNA (CT DNA) was purchased from Sigma Chemical Co. Ultrapure Milli-Q water (ρ > 18.0 MΩ cm) was used throughout the luminescence experiments. Ru(phen)3Cl2 used in experiments were synthesized according to the literature procedure.30Preparation of graphene nanosheets with different reducing agents
Graphite oxide (GO) was synthesized from natural flake graphite by a modified Hummers method.31 The as-prepared GO was dispersed in ultrapure water and sonicated for 60 min to achieve the exfoliation of GO dispersion (0.2 mg mL−1) for further use. Then, a serial of graphene nanosheets (GNS) were acquired using different reducing agents, such as hydrazine, glucose and urea, which were abbreviated as GNS-H, GNS-G and GNS-U, respectively.GNS-H was made followed the literature procedure.14 In short, 1.4 mL of ammonia solution (25%, w/w) and 0.2 mL of hydrazine solution (25%, w/w) were added to 500 mL of the as-prepared exfoliation GO dispersion (0.2 mg mL−1). After being vigorously shaken for 10 minutes, the suspension was then refluxed at 95 °C for 1 h under continuous magnetic stirring. The resulting dispersion was obtained, which was abbreviated as GNS-H.
GNS-U was made followed the literature procedure.15 In short, 1.0500 g of urea was added to 500 mL of the exfoliation GO dispersion (0.2 mg mL−1). After being vigorously shaken for a few minutes, the suspension was then refluxed at 100 °C for 24 h under continuous magnetic stirring. The resulting dispersion was obtained and abbreviated as GNS-U.
GNS-G was made followed the literature procedure.16 In short, 1.6000 g of glucose was added to 500 mL of the exfoliation GO dispersion (0.2 mg mL−1). Then 2.8 mL of aqueous ammonia solution (25%, w/w) was added to the resulting dispersion. After being vigorously shaken for a few minutes, the suspension was then refluxed at 95 °C for 1 h under continuous magnetic stirring. The resulting stable dispersion was obtained and abbreviated as GNS-G.
Characterization
X-ray diffraction (XRD) analysis was carried out with a D/Max2550VB+/PC X-ray diffractometer with Cu Kα (λ = 0.15406 nm), using an operation voltage and current of 40 kV and 30 mA, respectively. The atomic force microscopy (AFM) image of the synthesized GNS samples deposited on a freshly cleaved mica surface was taken with NanoScope V in tapping mode. Transmission electron microscopy (TEM) images were collected using a JEM-2100 microscope working at 200 kV. Specimens for observation were prepared by dispersing the samples into alcohol by ultrasonic treatment and dropped on carbon–copper grids. The X-ray photoelectron spectroscopy (XPS) measurement was performed with an Axis Ultra, kratos (UK) spectrometer using Al Kα excitation radiation (1486 eV). The Raman spectra were taken at room temperature in the spectra range 400–4000 cm−1 using an ALMEGA-TM Raman spectrometer system. The spectra were recorded using a 532 nm argon ion laser. Fourier transform infrared (FT-IR) spectra were obtained on a Brucher EQUINX55 FT-IR spectrophotometer by a standard KBr disk method in the range 400–4000 cm−1. Specimens for observation were prepared by dispersing the samples into alcohol by ultrasonic treatment and dropped on carbon–copper grids. The absorption and emission spectra were collected using a Shimadzu 1750 UV-visible spectrometer and a RF-5301 fluorescence spectrometer (Japan), respectively.Luminescence experiments
Stock solution of CT DNA solution (6.72 × 10−4 g mL−1) was prepared by dissolving commercial CT DNA in ultrapure water. Stock solution of Ru(phen)3Cl2 (0.49 µM) was prepared in ultrapure water. Then the as-prepared GNS suspension (0.2 mg mL−1) was gradually added into Ru(phen)3Cl2 solution (3 mL) with stirring until the luminescence was almost quenched. Finally, an increasing amount of CT DNA solution was added until the highest luminescence intensity was reached. The sample was stirred for 5 s each time before the luminescence spectra were recorded. In all the titration experiments, the total volume was maintained not exceed 5% of the original volume.Results and discussion
X-ray diffraction and morphology analysis
The X-ray diffraction (XRD) patterns of GO, GNS-H, GNS-U and GNS-G are presented in Fig. 1. The as-prepared GO has a layered structure with a basal spacing of 0.82 nm, showing the complete oxidation of graphite into the graphite oxide.32 After reduction using different reducing agents, such as hydrazine, glucose and urea, the peak at 10.7° of GO completely disappears, and the obtained GNS samples all shows broad characteristics peak in range of 20–30°, which is corresponded to the (002) diffraction of graphene.33 The result suggests that the GO was all reduced to graphene after by treating with different reducing agents.The morphology of the as-prepared GNS samples was observed by TEM as shown in Fig. 2. It is seen that the obtained GNS samples all show the thin nanoplatelets shape with corrugation and scrolling image, which is consistent with the literature.34,35 The thickness of the GNS samples was further characterized by AFM analysis. The results indicated that the average thickness of the GNS-U, GNS-G and GNS-H is about 1.46 nm, 1.39 and 1.34 nm, respectively.16,35
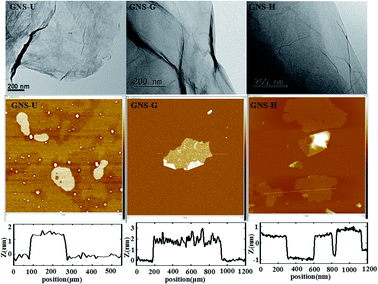 | ||
| Fig. 2 TEM and AFM images of GNS-U, GNS-G and GNS-H. And height profiles along the lines are shown in AFM images. | ||
The effective reduction of GO to GNS can be further demonstrated by the Raman technology. The Raman spectra of GO, GNS-H, GNS-U and GNS-G in the range from 1000 to 3000 cm−1 are shown in Fig. 3. The Raman spectrum of GO exhibits a weak D band at around 1361 cm−1 and a stronger G band at 1589 cm−1. In general, the G mode (∼1589 cm−1) is assigned to the E2g phonon of sp2 C atoms. While the D mode (∼1361 cm−1) is arisen from a breathing mode of κ-point phonons of A1g symmetry, which is a common feature of sp3 defects in carbon and usually can be associated with the structural defects, amorphous carbon, or edges that break the symmetry and selection rule.36,37 The ID/IG intensity ratio is a measure of the disorder/defects in graphene and average size of the sp2 domains in graphite materials.38 After reduction with hydrazine and glucose, the D band of the obtained GNS-H and GNS-G samples become prominent. The ID/IG intensity ratio for the samples of GNS-H, GNS-U and GNS-G is determined to be 1.31, 0.91 and 1.00, much higher than that of the pristine GO (0.76). The larger ID/IG value of the GNS samples prepared with different reducing agents indicates the significant decrease of the average size (or more amount) of the in-plane sp2 domains and more sp3 defects/disorders due to the reduction of GO to GNS.16,39–41 By comparison, GNS-H had the highest ID/IG intensity ratio (1.31), indicating the highest degree of reduction. GNS-G was the second, then GNS-U. The relative lower ID/IG intensity ratio (0.91) of the GNS-U sample can be assigned to the relative incomplete reduction of the GNS-U as compared to the sample of the GNS-G (1.00) and GNS-H (1.31).
IR spectra analysis
In this study, FTIR spectroscopy was also employed to evaluate the reduction of GO to GNS samples as shown in Fig. 4. The FTIR spectrum of GO exhibits the absorption bands at 3421 cm−1 (O–H stretching vibrations), 1717 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations from carbonyl and carboxylic groups), and 1617 cm−1 (C
O stretching vibrations from carbonyl and carboxylic groups), and 1617 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations). And the absorption bands at 1300–1000 cm−1 correspond to C–O stretching vibrations.42,43 After the reduction with hydrazine, urea and glucose, the absorption bands of GNS samples corresponding to oxygen functional groups C–O, and C
C stretching vibrations). And the absorption bands at 1300–1000 cm−1 correspond to C–O stretching vibrations.42,43 After the reduction with hydrazine, urea and glucose, the absorption bands of GNS samples corresponding to oxygen functional groups C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations were decreased significantly, indicating that most of oxygen functional groups on the GO nanosheets are removed.44 By comparison, for the sample of GNS-H, the absorption band at 1717 cm−1 (νC
O stretching vibrations were decreased significantly, indicating that most of oxygen functional groups on the GO nanosheets are removed.44 By comparison, for the sample of GNS-H, the absorption band at 1717 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) was indistinguishable, suggesting that the reduction effect with hydrazine was superior to that with glucose and urea. The IR spectra results are consistent with the Raman spectra analysis.
O) was indistinguishable, suggesting that the reduction effect with hydrazine was superior to that with glucose and urea. The IR spectra results are consistent with the Raman spectra analysis.
X-ray photoelectron spectroscopy analysis
XPS is one of the widely used techniques to characterize the removal of the oxygen groups in GNS. As shown in Fig. 5, the C1s XPS spectrum of GO (Fig. 5a) clearly shows a considerable degree of oxidation, with three different components corresponding to carbon atoms in different functional groups: the non-oxygenated ring C (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), epoxy and alkoxy carbon (C–O), and the carboxylate carbon (O–C
C), epoxy and alkoxy carbon (C–O), and the carboxylate carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).44–47 The peak of the non-oxygenated ring C (C–C) centered at 284.4 eV is attributed to bonds between sp2 hybridized carbon atoms. And two peaks located at 286.6 and 288.0 eV are assigned to the epoxy and alkoxy carbon (C–O) and the carboxylate carbon (O–C
O).44–47 The peak of the non-oxygenated ring C (C–C) centered at 284.4 eV is attributed to bonds between sp2 hybridized carbon atoms. And two peaks located at 286.6 and 288.0 eV are assigned to the epoxy and alkoxy carbon (C–O) and the carboxylate carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. After its reduction with hydrazine, urea and glucose, the C1s XPS spectra of the GNS samples (Fig. 5b–d) all exhibit these three types of carbon. However, compared to that of GO, the absorbance band intensities of the epoxy and alkoxy carbon (C–O) and the carboxylate carbon (O–C
O), respectively. After its reduction with hydrazine, urea and glucose, the C1s XPS spectra of the GNS samples (Fig. 5b–d) all exhibit these three types of carbon. However, compared to that of GO, the absorbance band intensities of the epoxy and alkoxy carbon (C–O) and the carboxylate carbon (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) for the GNS samples all decrease significantly, indicating the removal the oxygen-containing functional groups after the reduction.
O) for the GNS samples all decrease significantly, indicating the removal the oxygen-containing functional groups after the reduction.
The O/C ratio of the GO sample is 0.446 (Table 1), indicating a strong oxidation by using the modified Hummer method. After reduction, the O/C atomic ratio decreases from 0.446 (for GO) to 0.138 (for GNS-U), 0.118 (for GNS-G) and 0.110 (for GNS-H), respectively, indicating the efficient removal of oxygen functional groups.44,48,49 In contrast, the O/C ratio of GNS-H (0.110) is lower than that of GNS-U and GNS-G, demonstrating that the reduction degree was in the order of hydrazine > glucose > urea. The XPS results are consistent with the Raman and IR spectra analysis.
| Sample | Relative C (at%) | Relative O (at%) | O/C atomic ratio |
|---|---|---|---|
| GO | 69.18 | 30.82 | 0.446 |
| GNS-U | 87.86 | 12.14 | 0.138 |
| GNS-G | 89.48 | 10.52 | 0.118 |
| GNS-H | 90.11 | 9.89 | 0.110 |
In order to further investigate the interactions between GNS and Ru(phen)3Cl2, XPS spectra analysis of the GNS samples and GNS/Ru(phen)3Cl2 (Ru) were also done as shown in Fig. S2.† The C1s XPS spectra of the GNS samples all display a peak with binding energy (BE) of about 284.7 eV, which was assigned to C1s. As for the GNS/Ru samples, apart from the peak of C1s, the Ru3d components (Ru3d5/2 at 281.2 eV) can be also observed, suggesting the interaction between Ru(phen)3Cl2 and GNS.50–52 In particular, the peak of Ru3d3/2 were buried under the main C1s peak.52
UV-vis and luminescence spectra analysis
The UV-vis absorption spectra of the GNS samples and GNS/Ru(phen)3Cl2 (Ru) were shown in Fig. S3.† As shown in Fig. S3,† the UV-vis absorption spectra of the GNS samples in aqueous solution revealed the absorption bands at 260 nm for GNS-U, 264 nm for GNS-G, and 266 nm for GNS-H. The UV-vis spectrum of GO exhibits an absorption band at about 230 nm,16 while the absorption peak of the GNS samples all red shifted from 230 to 260–266 nm, indicating the deoxygenation of the GO and the electronic conjugation within the graphene sheets is restored after reduction with different reducing agents.53 In comparison, much greater red shifts are seen in this case of GNS-H than that of GNS-G and GNS-U, and the optical absorption of the GNS-H samples was the higher than that of the equal concentration of GNS-G and GNS-U, demonstrating the highest degree of reduction of GNS-H.16,53,54 The results are consistent with the Raman, IR spectra and XPS analysis. As for the GNS/Ru(phen)3Cl2 samples, apart from the absorption peaks of GNS at about 260–266 nm, there was also a new absorption peaks at about 222–226 nm, corresponding to the characteristic absorption of Ru(phen)3Cl2. Meanwhile, The absorption spectrum of GNS/Ru(phen)3Cl2 samples at about 222–226 nm slightly red-shifted compared with Ru(phen)3Cl2 at 221 nm, indicating the interaction between Ru(phen)3Cl2 and GNS.55The luminescence spectra of Ru(phen)3Cl2 (0.49 µM) in aqueous solution upon addition of different concentrations of the GNS samples are shown in Fig. 6. When the GNS samples were added gradually into Ru(phen)3Cl2 in aqueous solution, the luminescence intensity of Ru(phen)3Cl2 all systematically decreases as the GNS concentration increased (Fig. 6a, c and e), suggesting that strong π–π stacking interaction and electrostatic interaction existed between GNS samples and Ru(phen)3Cl2. When the concentration of GNS increases to a certain value, the luminescence intensity of Ru(phen)3Cl2 doesn't change any further. This means that no free Ru(phen)3Cl2 is left in the solution at this point. Among the GNS samples, GNS-G exhibits the most pronounced quenching efficiency (Fig. 6e), suggesting much stronger interactions existed between GNS-G and Ru(phen)3Cl2. Then CT DNA was added gradually into the above mentioned solutions. Along with the addition of CT DNA, the luminescence intensities of the above three system with GNS-H, GNS-U and GNS-G were all increased gradually as shown in Fig. 6b, d and f, respectively. After a certain concentration of DNA was added into the above mentioned system, the luminescence intensity was all fully recovered. The reason is that after being encountered with CT DNA, Ru(phen)3Cl2 was released from GNS and interacted with CT DNA immediately, leading to a luminescence recovery of Ru(phen)3Cl2.26,28 By comparison, the luminescence intensity of GNS-G was increased by 49-fold (Fig. S2c†), which is far beyond that of GNS-H (only 7 times increasing as shown in Fig. S4a†) and GNS-U (only 6 times increasing as shown in Fig. S4b†).
As shown in Fig. S5a,† Ru(phen)3Cl2 alone exhibited bright red luminescence under UV irradiation (Fig. S5a†). When the corresponding GNS samples were added into Ru(phen)3Cl2, the red luminescence of the Ru(phen)3Cl2 was significantly quenched (Fig. S5b–d†). Among the GNS samples, GNS-H exhibits the most pronounced quenching efficiency, and GNS-G was the second, then GNS-U. The result is consistent with the luminescence response as shown in Fig. 6a, c and e. The blue luminescence was emitted by GNS-G itself, which is consistent with the literatures.56,57 After a certain concentration of DNA was added into the above mentioned system, the red luminescence was all recovered (Fig. S5e–g†), which is consistent with the luminescence spectra analysis as shown in Fig. 6b, d and f. The reason is that after being encountered with CT DNA, Ru(phen)3Cl2 was released from GNS and interacted with CT DNA immediately, leading to a luminescence recovery of Ru(phen)3Cl2.
By comparison, the luminescence response of GNS-G (Fig. 7) shows the best linear correlation to the DNA added over the wide concentration range from 5.4 to 35.4 µg mL−1, with a correlation coefficient of 0.9971. The detection limit (3σ/k method)58 was 3.62 × 10−9 g mL−1, which is lower than the literature.59 It indicates that the GNS-G sample prepared with glucose can be employed as a carrier of Ru(phen)3Cl2 sensor to selective discriminate DNA. The results indicated that although the GNS samples prepared with different reductants can effectively quench the emission of the Ru(phen)3Cl2 sensor. After addition of a certain amount of DNA into the three systems, the luminescence intensity was all fully recovered. By comparison, the luminescence response of GNS-G prepared with glucose shows the best linear correlation to the DNA added. Therefore, the reduction degree of GNS is just one of the main factors on the sensing performance. The reason why GNS-G shows the strongest variation in luminescence intensity may be its better dispersion, better biocompatible property, better exfoliation and minimum restacking.16,60
 | ||
| Fig. 7 A calibration curve of GNS-H, GNS-U and GNS-G between the luminescence change at 590 nm and DNA added. | ||
Conclusions
Herein, a serial of GNS were fabricated using different reducing agents, such as hydrazine, glucose and urea. The reduction degrees and properties of the obtained GNS samples were systematically investigated by X-ray diffractometer, Raman spectra, IR spectra and X-ray photoelectron spectroscopy. The results indicated that the reduction degree with hydrazine was the highest. And glucose was the second, then urea. Therefore, reducing agents plays an important role in the bulk fabrication of high quality graphene. The GNS samples were all employed as a carrier of Ru(phen)3Cl2 sensor to discriminate DNA. It is found that all the GNS samples can effectively quench the emission of the Ru(phen)3Cl2 sensor. After the addition of a certain amount of DNA into the three systems, the luminescence intensity was all fully recovered. By comparison, the luminescence response of GNS-G prepared with glucose shows the best linear correlation to the DNA added, with a detection limit of 3.62 × 10−9 g mL−1, indicating GNS-G can be employed as a good carrier of Ru(phen)3Cl2 to discriminate DNA.In this work, the reduction degrees and properties of GNS prepared with different reducing agents were firstly systematically studied. And the feasible applications of GNS prepared with different reducing agents as a carrier of luminescent sensor were also systematically studied. It is believed that this work would advance the research of bulk fabrication of high quality graphene and the specific applications in luminescent sensor of the graphene-based functional materials in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 201205095), the Scientific Research Foundation of Northwest A&F University (Z109021115, Z111021103 and Z111021107), the Fundamental Research Funds for the Central Universities (Z109021204), Shaanxi Province Science and Technology (no. 2013K12-03-23), State Key Laboratory of Chemo/Biosensing and Chemometrics, Hunan University (no. 2013005), Open Project of Key Laboratory of Applied Surface and Colloid Chemistry (no. 2014012).Notes and references
- X. Y. Dong, L. Wang, D. Wang, C. Li and J. Jin, Langmuir, 2012, 28, 293 CrossRef CAS PubMed.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef CAS PubMed.
- Z. C. Xing, Q. X. Chu, X. B. Ren, J. Q. Tian, A. M. Asiri, K. A. Alamry, A. O. Al-Youbi and X. P. Sun, Electrochem. Commun., 2013, 32, 9 CrossRef CAS PubMed.
- H. Y. Li, S. Liu, J. Q. Tian, L. Wang, W. B. Lu, Y. L. Luo, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, ChemCatChem, 2012, 4, 1079 CrossRef CAS.
- Y. W. Zhang, J. Q. Tian, H. Y. Li, L. Wang, X. Y. Qin, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, Langmuir, 2012, 28, 12893 CrossRef CAS PubMed.
- D. Chen, L. H. Tang and J. H. Li, Chem. Soc. Rev., 2010, 39, 3157 RSC.
- Y. X. Liu, X. C. Dong and P. Chen, Chem. Soc. Rev., 2012, 41, 2283 RSC.
- A. Marinkas, F. Arena, J. Mitzel, G. M. Prinz, A. Heinzel, V. Peinecke and H. Natter, Carbon, 2013, 58, 139 CrossRef CAS PubMed.
- G. Q. Xu, P. W. Xu, D. J. Shi and M. Q. Chen, RSC Adv., 2014, 4, 28807 RSC.
- W. H. Shang, X. Y. Zhang, M. Zhang, Z. T. Fan, Y. Sun, M. Han and L. Z. Fan, Nanoscale, 2014, 6, 5799 RSC.
- S. Liu, J. Q. Tian, L. Wang and X. P. Sun, Carbon, 2011, 49, 3158 CrossRef CAS PubMed.
- S. Liu, J. Q. Tian, L. Wang, H. L. Li, Y. W. Zhang and X. P. Sun, Macromolecules, 2010, 43, 10078 CrossRef CAS.
- Z. J. Fan, W. Kai, J. Yan, T. Wei, L. J. Zhi, J. Feng, Y. M. Ren, L. P. Song and F. Wei, ACS Nano, 2011, 5, 191 CrossRef CAS PubMed.
- L. J. Deng, G. Zhu, J. F. Wang, L. P. Kang, Z. H. Liu, Z. P. Yang and Z. L. Wang, J. Power Sources, 2011, 196, 10782 CrossRef CAS PubMed.
- L. J. Zhang, X. G. Zhang, L. F. Shen, B. Gao, L. Hao, X. J. Lu, F. Zhang, B. Ding and C. Z. Yuan, J. Power Sources, 2012, 199, 395 CrossRef CAS PubMed.
- C. Z. Zhu, S. J. Guo, Y. X. Fang and S. J. Dong, ACS Nano, 2010, 4, 2429 CrossRef CAS PubMed.
- M. Quintana, E. Vazquez and M. Prato, Acc. Chem. Res., 2013, 46, 138 CrossRef CAS PubMed.
- F. X. Xiao, J. W. Miao and B. Liu, J. Am. Chem. Soc., 2014, 136, 1559 CrossRef CAS PubMed.
- H. L. Li, Y. W. Zhang, L. Wang, J. Q. Tian and X. P. Sun, Chem. Commun., 2011, 47, 961 RSC.
- H. L. Li, J. Q. Tian, L. Wang, Y. W. Zhang and X. P. Sun, J. Mater. Chem., 2011, 21, 824 RSC.
- S. Liu, H. L. Li, L. Wang, J. Q. Tian and X. P. Sun, J. Mater. Chem., 2011, 21, 339 RSC.
- H. L. Li, Y. W. Zhang, T. S. Wu, S. Liu, L. i. Wang and X. P. Sun, J. Mater. Chem., 2011, 21, 4663 RSC.
- H. L. Li, Y. W. Zhang, Y. L. Luo and X. P. Sun, Small, 2011, 7, 1562 CrossRef CAS PubMed.
- B. J. Hong, O. C. Compton, Z. An, I. Eryazici and S. T. Nguyen, ACS Nano, 2012, 6, 63 CrossRef CAS PubMed.
- J. Kim, L. J. Cote, F. Kim and J. Huang, J. Am. Chem. Soc., 2010, 132, 260 CrossRef CAS PubMed.
- A. B. Tossi and J. M. Kelly, Photochem. Photobiol., 1989, 49, 545 CrossRef CAS PubMed.
- C. Hiort, B. Nordén and A. Rodger, J. Am. Chem. Soc., 1990, 112, 1971 CrossRef CAS.
- D. Z. M. Coggan, I. S. Haworth, P. J. Bates, A. Robinson and A. Rodger, Inorg. Chem., 1999, 38, 4486 CrossRef CAS PubMed.
- H. J. Li, F. Y. Liu, S. G. Sun, J. Y. Wang, Z. Y. Li, D. Z. Mu, B. Qiao and X. J. Peng, J. Mater. Chem. B, 2013, 1, 4146 RSC.
- P. A. Lay, A. M. Sargeson, H. Taube, M. H. Chou and C. Creutz, Inorg. Synth., 1986, 24, 291 CrossRef CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Z. H. Liu, Z. M. Wang, X. J. Yang and K. Ooi, Langmuir, 2002, 18, 4926 CrossRef CAS.
- X. Wang, S. Zhou, W. Y. Xing, B. Yu, X. M. Feng, L. Song and Y. Hu, J. Mater. Chem. A, 2013, 1, 4383 CAS.
- Y. Y. Shao, J. Wang, M. Engelhard, C. M. Wang and Y. H. Lin, J. Mater. Chem., 2010, 20, 743 RSC.
- Y. C. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679 CrossRef CAS PubMed.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126 CrossRef CAS PubMed.
- A. C. Ferrari and J. Robertson, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 14095 CrossRef CAS.
- C. Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2009, 9, 2206 CrossRef.
- S. Stankovich, A. A. Dikin, R. D. Piner, K. A. Kohlhass, A. Kleinhammes, Y. Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS PubMed.
- G. X. Wang, J. Yang, J. S. Park, X. L. Gou, B. Wang, H. Liu and J. Yao, J. Phys. Chem. C, 2008, 112, 8192 CAS.
- G. Q. Luo, X. J. Jiang, M. J. Li, Q. Shen, L. M. Zhang and H. G. Yu, ACS Appl. Mater. Interfaces, 2013, 5, 2161 CAS.
- Y. Li, N. Q. Zhao, C. S. Shi, E. Z. Liu and C. N. He, J. Phys. Chem. C, 2012, 116, 25226 CAS.
- W. Zhang, Y. X. Zhang, Y. Tian, Z. Y. Yang, Q. Q. Xiao, X. Guo, L. Jing, Y. F. Zhao, Y. M. Yan, J. S. Feng and K. N. Sun, ACS Appl. Mater. Interfaces, 2014, 6, 2248 CAS.
- K. Liu, L. Chen, Y. Chen, J. L. Wu, W. Y. Zhang, F. Chen and Q. Fu, J. Mater. Chem., 2011, 21, 8612 RSC.
- D. Briggs and G. Beamson, The Scienta ESCA300 Database, John Wiley and Sons, New York, 1992 Search PubMed.
- X. Wang, S. Zhou, W. Y. Xing, B. Yu, X. M. Feng, L. Song and Y. Hu, J. Mater. Chem. A, 2013, 1, 4383 CAS.
- X. J. Zhou, J. J. Zhang, H. X. Wu, H. J. Yang, J. Y. Zhang and S. W. Guo, J. Phys. Chem. C, 2011, 115, 11957 CAS.
- Y. W. Zhu, M. D. Stoller, W. W. Cai, A. Velamakanni, R. D. Piner, D. Chen and R. S. Ruoff, ACS Nano, 2010, 4, 1227 CrossRef CAS PubMed.
- D. Y. Wan, C. Y. Yang, T. Q. Lin, Y. F. Tang, M. Zhou, Y. J. Zhong, F. Q. Huang and J. H. Lin, ACS Nano, 2012, 6, 9068 CrossRef CAS PubMed.
- U. Unal, Y. Matsumoto, N. Tanaka, Y. Kimura and N. Tamoto, J. Phys. Chem. B, 2003, 107, 12680 CrossRef CAS.
- Y. V. Larichev, J. Phys. Chem. C, 2008, 112, 14776 CAS.
- L. M. Martínez-Prieto, S. Carenco, C. H. Wu, E. Bonnefille, S. Axnanda, Z. Liu, P. F. Fazzini, K. Philippot, M. Salmeron and B. Chaudret, ACS Catal., 2014, 4, 3160 CrossRef.
- L. X. Lin and S. W. Zhang, J. Mater. Chem., 2012, 22, 14385 RSC.
- V. H. Pham, H. D. Pham, T. T. Dang, S. H. Hur, E. J. Kim, B. S. Kong, S. Kim and J. S. Chung, J. Mater. Chem., 2012, 22, 10530 RSC.
- G. F. Gui, Y. Zhuo, Y. Q. Chai, N. Liao, M. Zhao, J. Han, Y. Xiang and R. Yuan, RSC Adv., 2014, 4, 1955 RSC.
- Y. Q. Dong, G. L. Li, N. Zhou, R. X. Wang, Y. W. Chi and G. N. Chen, Anal. Chem., 2012, 84, 8378 CrossRef CAS PubMed.
- J. H. Shen, Y. H. Zhu, C. Chen, X. L. Yang and C. Z. Li, Chem. Commun., 2011, 47, 2580 RSC.
- X. B. Zhang, Z. D. Wang, H. Xing, Y. Xiang and Y. Lu, Anal. Chem., 2010, 82, 5005 CrossRef CAS PubMed.
- L. P. Wang, C. C. Guo, B. Fu and L. Wang, J. Agric. Food Chem., 2011, 59, 1607 CrossRef CAS PubMed.
- O. Akhavan, E. Ghaderi, S. Aghayee, Y. Fereydoonia and A. Talebi, J. Mater. Chem., 2012, 22, 13773 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The structures of Ru(phen)3Cl2; C1s XPS spectra of the GNS samples and GNS/Ru(phen)3Cl2 (Ru); UV-vis absorption spectra of the GNS samples and GNS/Ru. Luminescence response of the Ru(phen)3Cl2 sensor upon addition of different concentration of CT DNA in the presence of GNS; optical image of Ru(phen)3Cl2 (Ru), Ru + GNS and Ru + GNS + DNA. See DOI: 10.1039/c5ra02376a |
| This journal is © The Royal Society of Chemistry 2015 |





