Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Colorimetric and optical discrimination of halides by a simple chemosensor†
Syed A.
Haque
a,
Robert L.
Bolhofner
a,
Bryan M.
Wong
*b and
Md. Alamgir
Hossain
*a
aDepartment of Chemistry and Biochemistry, Jackson State University, Jackson, MS 39217, USA. E-mail: alamgir.hossain@jsums.edu
bDepartment of Chemical & Environmental Engineering, Materials Science & Engineering Program, University of California-Riverside, Riverside, CA 92521, USA. E-mail: bryan.wong@ucr.edu
First published on 21st April 2015
Abstract
A thiophene-based tripodal copper(II) complex has been synthesized as a new colorimetric and optical chemosensor for naked-eye discrimination of halides in acetonitrile and an acetonitrile–water mixture. The binding interactions of the new receptor with several anions were analyzed by UV-Vis titrations, electrospray ionization mass spectrometric (ESI-MS) experiments and density functional theory (DFT) calculations. The results from UV-Vis titrations indicate that the coordinative unsaturated copper(II) complex strongly binds a halide at its vacant copper(II) centre via a metal–ligand bond forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, exhibiting binding affinities in the order of fluoride > chloride > bromide > iodide. The interactions of the receptor with halides were further confirmed by ESI-MS, showing a distinct signal corresponding to a 1
1 complex, exhibiting binding affinities in the order of fluoride > chloride > bromide > iodide. The interactions of the receptor with halides were further confirmed by ESI-MS, showing a distinct signal corresponding to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex for each halide, suggesting that the noncovalent interactions also exist in the gas phase. In addition, time-dependent DFT (TD-DFT) calculations were also carried out to understand the excited-state properties of the chemosensor complexes. A detailed analysis of the TD-DFT calculations shows a consistent red-shift in the first optically-allowed transition, consistent with the observed colorimetric experiments.
1 complex for each halide, suggesting that the noncovalent interactions also exist in the gas phase. In addition, time-dependent DFT (TD-DFT) calculations were also carried out to understand the excited-state properties of the chemosensor complexes. A detailed analysis of the TD-DFT calculations shows a consistent red-shift in the first optically-allowed transition, consistent with the observed colorimetric experiments.
Introduction
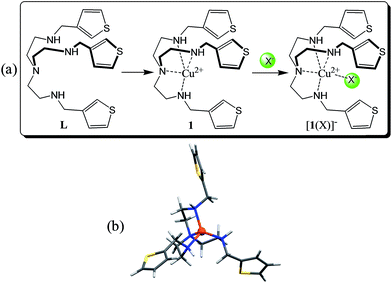
Anion recognition with synthetic receptors remains a frontier research area in chemical science due to its importance in several chemical, biological, and environmental systems.1,2 Because of their prevalence in these various applications, there is an acute need to design sensitive and selective receptors for the detection and monitoring of anions.3 Over the past several years this area has produced a diverse variety of molecular receptors4 that interact with anions through various non-covalent interactions such as hydrogen bonding,4–6 electrostatic,7–9 Lewis acid–base,10 and metal–ligand11–13 bonds. In particular, designed receptors with integrated sensing groups have recently received considerable attention in anion binding chemistry as they strongly and selectively interact with certain anions via reversible non-covalent interactions under a variety of conditions.14–16 Indeed, optical sensing is a quick and cost-effective method to detect an analyte without the use of expensive instruments that often require time-consuming sample preparation.16Certain receptors incorporated with transition metal ions such as copper,17–23nickel,24 zinc,25–27 and ruthenium28,29 often respond to an analyte by displaying distinct color or optical changes through metal–anion interactions in a solution under neutral conditions. Fabbrizzi and coworkers reported a furan-based dinuclear copper complex leading to the selective binding of chloride in water.18 Delgado and coworkers synthesized a para-xylyl-based dinuclear copper(II) complex that was found to bridge an oxalate or succinate between the two copper centers.22 Stang, Chi, and coworkers incorporated ruthenium ions into metallo-bowls to obtain tetranuclear complexes showing strong affinities for carboxylate anions in methanol.29 Beer and coworkers reported zinc-containing porphyrin-cages for the optical sensing of halides and oxoanions in acetone–water solvent mixtures.25 In our efforts to develop simple optical sensors for anions, we have previously synthesized N-methyl-2,2′-diaminodiethylamine-driven dinuclear copper complexes for the selective binding of iodide,30 phosphate,31 and cyanide.32 Previous studies have demonstrated that coordinatively unsaturated copper(II) complexes can effectively be used for the detection of certain anions via metal–anion interactions.18,22,30–33 Recently, Caltagirone and Lippolis and coworkers have incorporated a copper(II) ion into a tetradentate ligand derived from a quinoline-based triazacyclononane. This was subsequently used to synthesize an unsaturated copper(II) complex that was shown to form a penta-coordinated complex with iodide or cyanide, displaying visible color changes.34 Our continuing efforts in designing highly sensitive optical receptors led us to explore a copper(II) complex with a simple and readily obtainable tripodal-based tetradentate ligand (L) for anions. Herein, we report a simple tripodal-based copper(II) complex 1 integrated with 3-thiophene spacers for the naked-eye discrimination of halides in acetonitrile.
Results and discussion
Synthesis
The synthesis of L was straightforward and accomplished through a condensation reaction of tren with 3-thiophenecarboxaldehyde providing a high yield. The copper(II) complex 1 was obtained as a blue powder by mixing of L and copper nitrate in CH3CN at room temperature, yielding a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
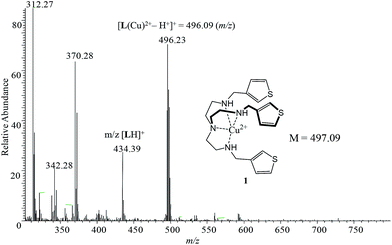
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 copper complex as [Cu(L)](NO3)2. It is assumed that the copper is coordinatively unsaturated and is bonded with four nitrogen atoms as shown in Scheme 1. The two nitrates serve as outer sphere ligands to balance the positive charges of copper in the complex. This assumption was further confirmed by DFT calculations of 1 showing tetracoordinated copper(II) in a trigonal pyramidal environment (Scheme 1b) and by MS-ESI (+ve) displaying a m/z = 496.23 which corresponds to a [Cu(L) − H+]+ moiety (Fig. 1). Thus, this complex has the potential to bind an anion to its metal center. A similar coordination sphere at the copper(II) center was previously reported in the literature.33,34
1 copper complex as [Cu(L)](NO3)2. It is assumed that the copper is coordinatively unsaturated and is bonded with four nitrogen atoms as shown in Scheme 1. The two nitrates serve as outer sphere ligands to balance the positive charges of copper in the complex. This assumption was further confirmed by DFT calculations of 1 showing tetracoordinated copper(II) in a trigonal pyramidal environment (Scheme 1b) and by MS-ESI (+ve) displaying a m/z = 496.23 which corresponds to a [Cu(L) − H+]+ moiety (Fig. 1). Thus, this complex has the potential to bind an anion to its metal center. A similar coordination sphere at the copper(II) center was previously reported in the literature.33,34
UV-Vis binding studies
The receptor 1 showed an intense blue color in CH3CN. The addition of one equivalent of F−, Cl−, Br−, and I− to the receptor (5 × 10−3 M in CH3CN) showed a distinct visual color change for each halide, indicating strong interactions between the halides and 1. As shown in Fig. 2, the blue color of the solution of 1 changed to aqua for fluoride, lime for chloride, turquoise for bromide, and greenish-yellow for iodide. On the other hand, the color remained the same after the addition of NO3− and ClO4−, suggesting that 1 can be used to effectively discriminate halides in CH3CN. The receptor was also examined in a mixture of CH3CN–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), showing a noticeable color change for only iodide (Fig. 3), as also previously observed with a dinuclear copper(II) complex.30
1, v/v), showing a noticeable color change for only iodide (Fig. 3), as also previously observed with a dinuclear copper(II) complex.30
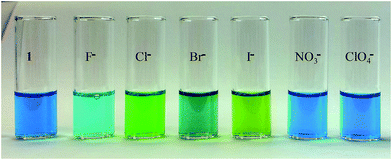 | ||
| Fig. 2 Colorimetric studies of anions (F−, Cl−, Br−, I−, NO3−, and ClO4−) with 1 in CH3CN at room temperature. | ||
 | ||
Fig. 3 Colorimetric studies of anions (F−, Cl−, Br−, I−, NO3−, and ClO4−) with 1 in CH3CN–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at room temperature. 1, v/v) at room temperature. | ||
The binding properties of 1 were then investigated by UV-Vis spectroscopy in CH3CN using [n-Bu4N]+ salts of halides. As shown in Fig. 4, the complex showed an absorption band at 291 nm in the absence of an anion. Upon the addition of one equivalent of different halides, the absorption pattern of 1 changed significantly for each halide with respect to the intensity and absorption maximum. Such a change is attributed to the possible axial ligation of a halide anion to the coordinatively unsaturated copper center leading to the formation of a metal–halide bond. This also accounts for the color change of 1 due to the addition of halides. A significant increase in the absorption intensity was observed for chloride and bromide, while an obvious red shift in the absorption maximum was observed in the presence of bromide (291 to 310 nm), and a blue shift for fluoride (291 to 270 nm). For iodide, there was a decrease in the absorption maxima, while two new peaks appeared at longer wavelengths. The shortest absorption band for fluoride at 270 nm compared to that for iodide at 436 nm indicates that the strong ligand field-effect of F− lowers the eg energy level of copper(II), thus resulting in the transition at higher energy. However, there was no change in the absorption pattern of 1 after the addition of nitrate or perchlorate.
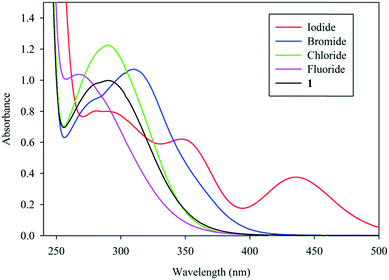 | ||
| Fig. 4 Changes in the absorbance of 1 (1 × 10−4 M) in the presence of one equivalent of different halides in CH3CN at room temperature. λmax: 1 = 291, I− = 290, Br− = 310, Cl− = 289, F− = 270 nm. | ||
The binding properties of the receptor for halides were determined by UV-Vis titration methods in CH3CN. As shown in Fig. 5a, the incremental addition of F− to 1 in CH3CN resulted in a gradual hypsochromic (blue) shift of the absorption maxima (291 to 262 nm), while the absorption intensities were gradually decreased, showing an isosbestic point at 271 nm. Interestingly, in the case of bromide, a bathochromic (red) shift in the absorbance band (291 to 310 nm) was observed with a gradual increase in the absorption intensity, displaying an isosbestic point at 296 nm (Fig. 5b). In both cases, the isosbestic point is observed at an equimolar ratio of the receptor and the respective halide, suggesting the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (receptor
1 (receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) halide) complex. The absorption intensity of 1 was notably enhanced (hyperchromic shift) after the gradual addition of chloride to the receptor, while the absorption maxima (λmax) remained almost unchanged (Fig. 5c). On the other hand, the absorption pattern of the receptor was quite different upon the gradual addition of iodide. New bands in the 330 to 390 nm region as well as strong Soret bands in the 400–500 nm region are shown (Fig. 5d), which could be due to the formation of a charge transfer complex.13,29
halide) complex. The absorption intensity of 1 was notably enhanced (hyperchromic shift) after the gradual addition of chloride to the receptor, while the absorption maxima (λmax) remained almost unchanged (Fig. 5c). On the other hand, the absorption pattern of the receptor was quite different upon the gradual addition of iodide. New bands in the 330 to 390 nm region as well as strong Soret bands in the 400–500 nm region are shown (Fig. 5d), which could be due to the formation of a charge transfer complex.13,29
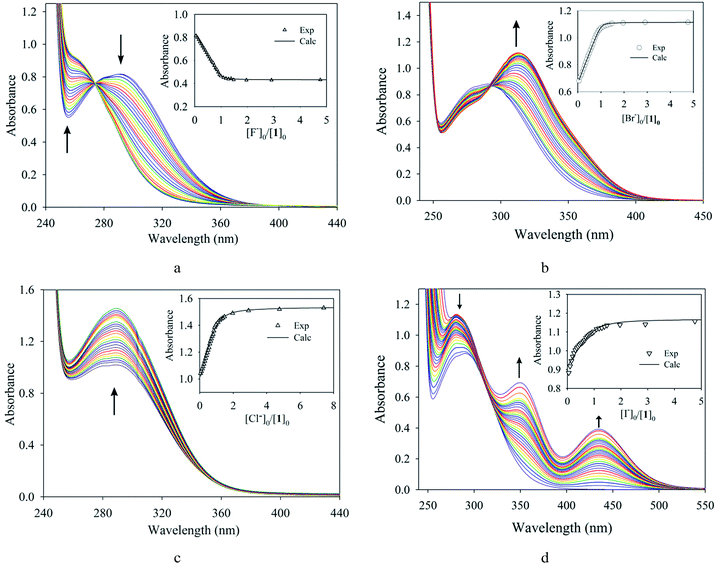 | ||
| Fig. 5 Changes in the absorption spectra of 1 (1 × 10−4 M) with an increasing amount of (a) fluoride, (b) bromide, (c) chloride, and (d) iodide in CH3CN. The titration curves are shown in the insets. | ||
A Job plot analysis confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host
1 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest binding stoichiometry of halide binding to 1 for each halide, showing a maximum at a 0.5 mole fraction (Fig. 6 and S6–S8 ESI†). The halide binding constants (K) were determined by a non-linear regression of the absorbance changes of the receptor based on a 1
guest binding stoichiometry of halide binding to 1 for each halide, showing a maximum at a 0.5 mole fraction (Fig. 6 and S6–S8 ESI†). The halide binding constants (K) were determined by a non-linear regression of the absorbance changes of the receptor based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (insets in Fig. 5),35 and are reported in Table 1. The receptor binds halide anions strongly, showing the highest association constant for fluoride with a strong selectivity over other halides. This is presumably a result of a stronger field-effect of the tiny fluoride,36 and is possibly bound at the vacant site of the metal center of 1 (Scheme 1). The binding trend of the receptor correlates directly with the ligand field-effect36 and the charge density of anions in the order of F− > Cl− > Br− > I−. This binding trend is consistent with the results reported by Beer and coworkers for triazolium-containing zinc(II) metalloporphyrins,25 and by us with macrocycle-based receptors appended with dansyl groups.37 However, an opposite trend was observed with a macrocycle-based copper(II) complex, where the size complementarily dominated over charge density.301 was also tested for other anions including sulfate, phosphate, acetate, cyanide, benzoate, hydroxide, pyrophosphate, and citrate in CH3CN. The receptor was found to bind acetate, hydroxide, and sulfate showing the binding constants (in log
1 binding model (insets in Fig. 5),35 and are reported in Table 1. The receptor binds halide anions strongly, showing the highest association constant for fluoride with a strong selectivity over other halides. This is presumably a result of a stronger field-effect of the tiny fluoride,36 and is possibly bound at the vacant site of the metal center of 1 (Scheme 1). The binding trend of the receptor correlates directly with the ligand field-effect36 and the charge density of anions in the order of F− > Cl− > Br− > I−. This binding trend is consistent with the results reported by Beer and coworkers for triazolium-containing zinc(II) metalloporphyrins,25 and by us with macrocycle-based receptors appended with dansyl groups.37 However, an opposite trend was observed with a macrocycle-based copper(II) complex, where the size complementarily dominated over charge density.301 was also tested for other anions including sulfate, phosphate, acetate, cyanide, benzoate, hydroxide, pyrophosphate, and citrate in CH3CN. The receptor was found to bind acetate, hydroxide, and sulfate showing the binding constants (in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K) of 4.22(2), 4.00(2) and 3.65(2), respectively. However, the titration for other anions was hampered due to the precipitation during the titration process. The change of absorbance (ΔA) with the concentration of a halide shows a linear dependency up to 150 μM in CH3CN, allowing us to estimate the lowest detection limit (LOD) up to 3.01, 5.24, 9.72, and 15.6 μM for fluoride, chloride, bromide, and iodide, respectively (Fig. S13 and Table S1 in ESI†).38
K) of 4.22(2), 4.00(2) and 3.65(2), respectively. However, the titration for other anions was hampered due to the precipitation during the titration process. The change of absorbance (ΔA) with the concentration of a halide shows a linear dependency up to 150 μM in CH3CN, allowing us to estimate the lowest detection limit (LOD) up to 3.01, 5.24, 9.72, and 15.6 μM for fluoride, chloride, bromide, and iodide, respectively (Fig. S13 and Table S1 in ESI†).38
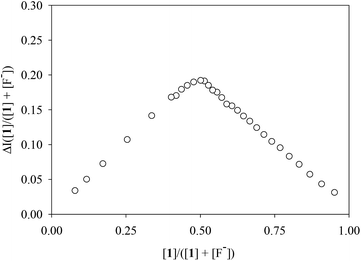 | ||
| Fig. 6 Job plot analysis of 1 for the binding of fluoride in CH3CN. The change of the absorbance (ΔI) of 1 was determined from the titration plot as shown in Fig. 5a. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K) of 1 with halides
K) of 1 with halides
| Anion | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K K |
|
|---|---|---|
| CH3CN | CH3CN–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
|
| Fluoride | 5.83(3) | 4.58(3) |
| Chloride | 4.86(2) | 4.18(2) |
| Bromide | 4.80(2) | 4.16(2) |
| Iodide | 4.72(2) | 3.98(2) |
We also performed titration studies of 1 for halides in an aqueous medium using the mixture of CH3CN–H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The receptor was found to bind a halide in a 1
1, v/v). The receptor was found to bind a halide in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode (Fig. S14†) showing a weaker binding trend than that observed in pure CH3CN (Table 1), which is due to the effect of increasing solvent polarity.30 The appearance of a new band at about 430 nm due to the addition of iodide (Fig. S14(d)†) also supports the color change for this anion in CH3CN–H2O (Fig. 3). The LOD for iodide was estimated to 24.9 μM in CH3CN–H2O (Fig. S15 and Table S3†) as compared to 15.5 μM in CH3CN.
1 binding mode (Fig. S14†) showing a weaker binding trend than that observed in pure CH3CN (Table 1), which is due to the effect of increasing solvent polarity.30 The appearance of a new band at about 430 nm due to the addition of iodide (Fig. S14(d)†) also supports the color change for this anion in CH3CN–H2O (Fig. 3). The LOD for iodide was estimated to 24.9 μM in CH3CN–H2O (Fig. S15 and Table S3†) as compared to 15.5 μM in CH3CN.
Mass spectrometry
Mass spectrometry is a powerful tool used to identify an ionic adduct at a low concentration.39–42 It is a rapid method for studying noncovalent bonds in a supramolecular complex, providing insights into the binding strength, sensitivity, and selectivity of host molecules complementary to that obtained from more traditional techniques. Kavallieratos and coworkers used an APCI-MS (atmospheric pressure chemical ionization mass spectrometry) for the detection of halides and nitrate with sulfonamides in dichloromethane.39 Our group recently used ESI-MS to probe a sulfate complex with a thiophene-based monocycle40 and an octameric phosphate cluster with a tren-based amine.41 This is a direct method to examine the stability and stoichiometry of a complex in a gaseous state. The results from UV-Vis titrations suggest that the positively charged receptor binds a halide forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex in CH3CN. We therefore used an ESI-MS in a positive ion detection mode using their halide salts in the same solvent, after the essential thermal desorption.
1 complex in CH3CN. We therefore used an ESI-MS in a positive ion detection mode using their halide salts in the same solvent, after the essential thermal desorption.
The free receptor shows a prominent signal at m/z = 496 that corresponds to the deprotonated receptor [1 − H+]+ (Fig. 1). After the addition of one equivalent of each halide, the complexes of [1 + F−]+, [1 + Cl−]+, [1 + Br−]+, and [1 + I−]+ at m/z = 516, 523, 578, and 624, respectively, were identified along with the deprotonated receptor [1 − H+]+ at m/z = 496 (Fig. 6). Strong intensities of mass-to-charge ratios were observed for [1 + Cl−]+ and [1 + Br−]+ complexes, while the corresponding signals for [1 + F−]+ and [1 + I−]+ were relatively weak. Similar strong signals for chloride complexes were reported earlier for ESI-MS by Cole and Zhu42 and for APCI-MS by Kavallieratos and coworkers.39 The signals corresponding to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of 1 with the respective halides are in agreement with the results of UV-Vis titrations in solution. These results further suggest that the noncovalent interactions also exist in the gaseous phase, and the adduct in solution-phase can be transferred into the gas-phase without interrupting the binding stoichiometry.
1 complexes of 1 with the respective halides are in agreement with the results of UV-Vis titrations in solution. These results further suggest that the noncovalent interactions also exist in the gaseous phase, and the adduct in solution-phase can be transferred into the gas-phase without interrupting the binding stoichiometry.
DFT calculations
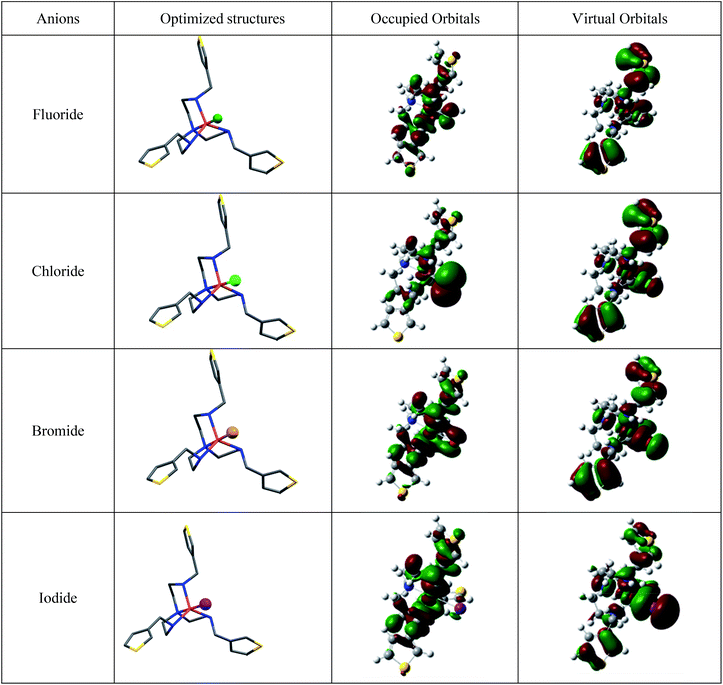
To quantitatively understand the interactions of the various copper halide molecules with the receptor, theoretical calculations based on density functional theory (DFT) were performed with the M06L meta-GGA functional.43An all-electron, polarized 6-31g(d,p) basis set was used for the copper atom, and a 6-311g(d,p) basis set was used for all of the other atomic species (H, C, N, S, F, Cl, Br, and I). Extensive previous work has shown that the M06L semi-local functional accurately predicts binding energies in both organometallic compounds44 as well as noncovalent interactions for large systems.45 Fully unconstrained geometry optimizations were carried out on both the isolated receptor as well as the various molecular-bound complexes. The DFT-optimized geometries of the complexes are shown in Fig. 7. With the optimized geometry, a binding energy was calculated with the expression: Ebinding = E[CuX]+ + E1 − E[1(X−)]+, where X− represents a F−, Cl−, Br−, or I−. Using this expression for each of the copper halide systems, we obtained attractive interactions for the halide complexes with binding energies in the order of [1(F)]− > [1(Cl)]− > [1(Br)]− > [1(I)]− (Table 2). This trend is in agreement with the binding constants determined experimentally by UV-Vis titrations. Notably, the magnitudes of the binding energies are proportional to the electronegativity of halides, with fluoride having the largest binding energy and iodide having the lowest binding.
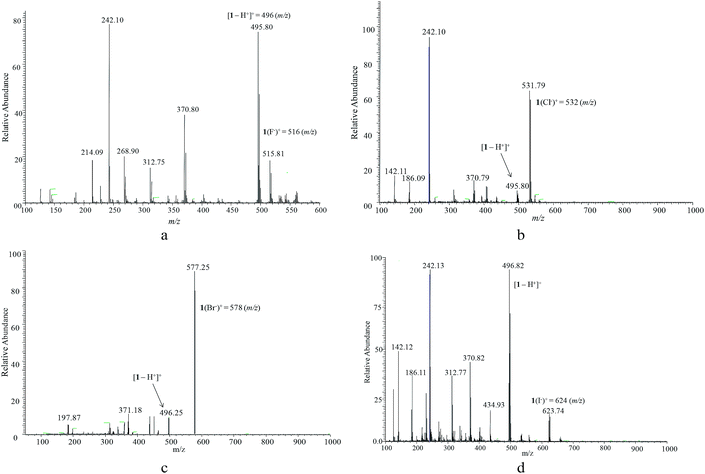 | ||
| Fig. 7 ESI-MS spectra of 1 in the presence of one equivalent of (a) fluoride, (b) chloride, (c) bromide, and (d) iodide in CH3CN. | ||
| Anion | E, kcal mol−1 | Excitation energy (eV) | Oscillator Strength |
|---|---|---|---|
| a Calculated with density functional theory (DFT) using the M06L meta-GGA functional. b Obtained at the TD-ωB97 level of theory. | |||
| Fluoride | −219.07 | 1.48 | 0.0030 |
| Chloride | −210.57 | 1.30 | 0.0028 |
| Bromide | −197.86 | 1.25 | 0.0026 |
| Iodide | −188.61 | 1.21 | 0.0023 |
To give further support to the observed colorimetric results, we also carried out high-level time-dependent density functional theory (TD-DFT) calculations on all of the copper-halide complexes. In order to account for charge-transfer effects in these complexes, we used the ωB97 range-separated functional which incorporates a full 100% asymptotic Hartree–Fock exchange. In our previous work on range-separated functionals, we46–49 and others50,51 have previously shown that maintaining a full 100% contribution of asymptotic Hartree–Fock exchange is essential for accurately describing valence excitations in even relatively simple molecular systems. Due to the size of the complexes and the large basis sets used, we only calculated the lowest 6 excited states for each complex. In addition, since each complex has an open-shell ground state, all the TD-DFT calculations were carried out with an unrestricted electronic configuration, which limited our study to only the lowest 6 excited states. All excited states and energies are given in the ESI, and Table S4† summarizes the excitation energies with the highest oscillator strengths for the various copper halide complexes.
Based on an analysis of the TD-ωB97 excitation energies, there is a consistent decrease in the first excited-state energy as one proceeds further down the periodic table. The predicted red-shift in the excitation energy for chloride, bromide, and iodide is consistent with the observed colorimetric experiments and highlights the utility of the TD-DFT calculations. The highest excitation energy for the fluoride complex accounts for the blue-shift of the UV-Vis absorbance spectrum (291 to 270 nm) observed experimentally. Fig. 8 displays both the occupied and virtual orbitals that contribute to the first excited-state transition for the various complexes. In each of these optically-allowed transitions, there is a re-arrangement of electron density from the copper halide orbitals to electron orbitals that are localized on the thiophene groups. It is interesting to note that, in each case, there is also some electronic re-arrangement in the excited state to σ orbitals on the halide atom, with the size of the σ orbital being proportional to the size of the halide atom.
Conclusions
We have reported a simple new tripodal copper(II) complex that is capable of discriminating halides via a distinct color change for each halide in acetonitrile. The new receptor was investigated for anions by UV-Vis titrations, electrospray ionization mass spectrometric (ESI-MS) experiments, and various computational methods. UV-Vis titration experiments reveal that the receptors exhibit strong anion binding affinities forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex with each halide in acetonitrile and acetonitrile–water mixture. Specifically, the receptor was shown to respond differently upon the addition of different halides with respect to the intensity and absorption maximum, which is attributed to an axial ligation of a halide anion to the coordinatively unsaturated copper center leading to the formation of a halide complex. The observed binding order of fluoride > chloride > bromide > iodide determined by UV-Vis titrations directly correlates with the relative basicity of the respective halide. The ESI-MS was also employed to identify the receptor–halide complex, showing a distinct signal for a 1
1 stoichiometric complex with each halide in acetonitrile and acetonitrile–water mixture. Specifically, the receptor was shown to respond differently upon the addition of different halides with respect to the intensity and absorption maximum, which is attributed to an axial ligation of a halide anion to the coordinatively unsaturated copper center leading to the formation of a halide complex. The observed binding order of fluoride > chloride > bromide > iodide determined by UV-Vis titrations directly correlates with the relative basicity of the respective halide. The ESI-MS was also employed to identify the receptor–halide complex, showing a distinct signal for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex for each halide. Furthermore, the DFT and TD-DFT calculations give additional insight in the electronic properties of this novel chemosensor. The ground-state DFT results corroborate and validate the binding energy trends observed experimentally for the various anions. Moreover, the excited-state TD-DFT calculations give detailed information on the oscillator strengths and orbitals involved in the first optically-allowed transition. The progressive absorbance red-shift as one proceeds down the periodic table is consistent with the observed colorimetric experiments and further highlights the utility of both experiment and predictive calculations for fully characterizing these chemosensors.
1 complex for each halide. Furthermore, the DFT and TD-DFT calculations give additional insight in the electronic properties of this novel chemosensor. The ground-state DFT results corroborate and validate the binding energy trends observed experimentally for the various anions. Moreover, the excited-state TD-DFT calculations give detailed information on the oscillator strengths and orbitals involved in the first optically-allowed transition. The progressive absorbance red-shift as one proceeds down the periodic table is consistent with the observed colorimetric experiments and further highlights the utility of both experiment and predictive calculations for fully characterizing these chemosensors.
Experimental
General
All reagents and solvents were purchased as reagent grade and used without further purification. Nuclear magnetic resonance (NMR) spectra were recorded on a Varian Unity INOVA 500 FT-NMR. Chemical shifts for samples were measured in CDCl3 or DMSO-d6 and calibrated with tetramethylsilane (TMS) as an internal reference. Elemental analysis was carried out using an ECS 4010 Analytical Platform (Costech Instrument) at Jackson State University. The absorbance was measured on a UV-2600 UV-VIS spectrophotometer (SHIMADZU). Mass spectral data were obtained in the ESI-MS positive mode on a TSQ Quantum GC (Thermo Scientific).Synthesis
UV titration studies
UV-Vis titration studies were performed by titrating 1 with [n-Bu4N]+A− in CH3CN at 25 °C. Initial concentrations of 1 and the anions were 5 × 10−5 M and 5 × 10−3 M, respectively. Each titration was performed by 20–25 measurements ([A−]0/[1]0 = 0–10 equivalents), and the binding constant (K) was calculated by fitting the change of UV-Vis absorbance (δI) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 association model using the equation, ΔI = ([A]0 + [1]0 + 1/K − ([A]0+ [1]0 + 1/K)2 − 4[1]0[A]0)1/2)ΔImax/Δ[1]0 (where 1 = receptor and A = anion).35 The error limit in K was less than 10%.
1 association model using the equation, ΔI = ([A]0 + [1]0 + 1/K − ([A]0+ [1]0 + 1/K)2 − 4[1]0[A]0)1/2)ΔImax/Δ[1]0 (where 1 = receptor and A = anion).35 The error limit in K was less than 10%.
ESI-MS experiments
Mass spectral data were obtained in the ESI-MS positive mode by a TSQ Quantum GC. The mass spectrometer was run for m/z = 0–1000 in CH3CN as a solvent. The pure solvent was run through the instrument before each analysis. Stock solutions (1 × 10−3 M) of 1 and each halide ([n-Bu4N]+X−) were prepared separately in CH3CN. Each sample was prepared by mixing of the host and respective halide solutions at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratios, and the mixture was further diluted to make a final concentration of 1 × 10−6 M. The resulting solution was introduced directly into the mass spectrometer by a micro syringe. A pure host solution of 1 × 10−6 M was also prepared, and its mass spectrum was run for a comparison.
1 volume ratios, and the mixture was further diluted to make a final concentration of 1 × 10−6 M. The resulting solution was introduced directly into the mass spectrometer by a micro syringe. A pure host solution of 1 × 10−6 M was also prepared, and its mass spectrum was run for a comparison.
Computational studies
Binding energies and structural optimization of copper complexes were evaluated with density functional theory (DFT) calculations,43 and optical properties were calculated using time-dependent density functional theory (TD-DFT) calculations (see Section 3.3 for further details). All the calculations were carried out using the Gaussian 09 package of programs.52Acknowledgements
The National Science Foundation is acknowledged for a CAREER award (CHE-1056927) to MAH. The NMR core facility at Jackson State University was supported by the National Institutes of Health (G12RR013459). R. L. B. was supported by NSF REU site (CHE 1156111) for undergraduate research at Jackson State University. S.A.H. acknowledges Jackson State University for a Graduate Assistantship Award (2014–2015). B.M.W. acknowledges the National Science Foundation for the use of supercomputing resources through the Extreme Science and Engineering Discovery Environment (XSEDE), Project no. TG-DMR140054.Notes and references
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry, John Wiley & Sons, Ltd., Hoboken, NJ, 2nd edn, 2009 Search PubMed.
- K. Bowman-James, A. Bianchi and E. García-Espana, Anion Coordination Chemistry, Wiley-VCH, New York, 2011 Search PubMed.
- Topics in current chemistry, ed. I. Stibor, Springer-Verlag, Berlin, 2005, vol. 255 Search PubMed.
- J. Yoon, S. K. Kim, N. J. Singh and K. S. Kim, Chem. Soc. Rev., 2006, 35, 355–360 RSC.
- Y. Hua and A. H. Flood, Chem. Soc. Rev., 2010, 39, 1262–1271 RSC.
- M. A. Hossain, R. A. Begum, V. W. Day and K. Bowman-James, in Supramolecular Chemistry: From Molecules to Nanomaterials, ed. P. A. Gale and J. W. Steed, John Wiley & Sons, Ltd., 2012 Search PubMed.
- E. García-España, P. Díaz, J. M. Llinares and A. Bianchi, Coord. Chem. Rev., 2006, 250, 2952–2986 CrossRef.
- M. Wenzel, J. R. Hiscock and P. A. Gale, Chem. Soc. Rev., 2012, 41, 480–520 RSC.
- M. A. Hossain, Curr. Org. Chem., 2008, 12, 1231–1256 CrossRef CAS.
- F. P. Schmidtchen and M. Berger, Chem. Rev., 1997, 97, 1609–1646 CrossRef CAS.
- E. J. O'Neil and B. D. Smith, Coord. Chem. Rev., 2006, 250, 3068–3080 CrossRef.
- L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 2013, 42, 1681–1699 RSC.
- P. Mateus, L. M. P. Lima and R. Delgado, Polyhedron, 2013, 52, 25–42 CrossRef CAS.
- L. Basabe-Desmonts, D. N. Reinhoudta and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993–1017 RSC.
- J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780–3799 CrossRef CAS.
- E. V. Anslyn, J. Org. Chem., 2007, 72, 687–699 CrossRef CAS.
- C. J. Harding, F. E. Mabbs, E. J. L. MacInnes, V. McKee and J. Nelson, J. Chem. Soc., Dalton Trans., 1996, 3227–3230 RSC.
- V. Amendola, E. Bastianello, L. Fabbrizzi, C. Mangano, P. Pallavicini, A. Perotti, A. M. Lanfredi and F. Ugozzoli, Angew. Chem., Int. Ed., 2000, 39, 2917–2920 CrossRef CAS.
- V. Amendola, G. Bergamaschi, A. Buttafava, L. Fabbrizzi and E. Monzani, J. Am. Chem. Soc., 2010, 132, 147–156 CrossRef CAS.
- J.-M. Chen, X.-M. Zhuang, L.-Z. Yang, L. Jiang, X.-L. Feng and T.-B. Lu, Inorg. Chem., 2008, 47, 3158–3165 CrossRef CAS.
- G. De Santis, L. Fabbrizzi, M. Licchelli, A. Poggi and A. Taglietti, Angew. Chem., Int. Ed. Engl., 1996, 35, 202–204 CrossRef CAS.
- S. Carvalho, R. Delgado, M. G. B. Drew and V. Félix, Dalton Trans., 2007, 2431–2439 RSC.
- F. Li, R. Delgado and V. Félix, Eur. J. Inorg. Chem., 2005, 4550–4561 CrossRef CAS.
- M. M. Rhaman, F. R. Fronczek, D. R. Powell and M. A. Hossain, Dalton Trans., 2014, 43, 4618–4621 RSC.
- L. C. Gilday, N. G. White and P. D. Beer, Dalton Trans., 2012, 41, 7092–7097 RSC.
- C.-H. Lee, S. Lee, H. Yoon and W.-D. Jang, Chem.–Eur. J., 2011, 17, 13898–13903 CrossRef CAS.
- M. P. Clares, J. Aguilar, R. Aucejo, C. Lodeiro, M. T. Albelda, F. Pina, J. C. Lima, A. J. Parola, J. Pina, J. S. de Melo, C. Soriano and E. García-España, Inorg. Chem., 2004, 43, 6114–6122 CrossRef CAS.
- V. Vajpayee, Y. H. Song, M. H. Lee, H. Kim, M. Wang, P. J. Stang and K.-W. Chi, Chem.–Eur. J., 2011, 17, 7837–7844 CrossRef CAS.
- A. Mishra, V. Vajpayee, H. Kim, M. H. Lee, H. Jung, M. Wang, P. J. Stang and K.-W. Chi, Dalton Trans., 2012, 41, 1195–1201 RSC.
- J. S. Mendy, M. A. Saeed, F. R. Fronczek, D. R. Powell and M. A. Hossain, Inorg. Chem., 2010, 49, 7223–7225 CrossRef CAS.
- M. A. Saeed, D. R. Powell and M. A. Hossain, Tetrahedron Lett., 2010, 51, 4904–4907 CrossRef CAS.
- M. M. Rhaman, A. Alamgir, B. M. Wong, D. R. Powell and M. A. Hossain, RSC Adv., 2014, 4, 54263–54267 RSC.
- N. Singh, H. J. Jung and D. O. Jang, Tetrahedron Lett., 2009, 50, 71–74 CrossRef CAS.
- M. A. Tetilla, M. C. Aragoni, M. Arca, C. Caltagirone, C. Bazzicalupi, A. Bencini, A. Garau, F. Isaia, A. Laguna, V. Lippolis and V. Meli, Chem. Commun., 2011, 47, 3805–3807 RSC.
- H. J. Schneider, R. Kramer, S. Simova and U. Schneider, J. Am. Chem. Soc., 1988, 110, 6442–6448 CrossRef CAS.
- G. L. Miessler and D. A. Tarr, in Inorganic Chemistry, Pearson Prentice Hall, 3rd edn, 2003, p. 368 Search PubMed.
- K. R. Dey, B. M. Wong and M. A. Hossain, Tetrahedron Lett., 2010, 51, 1329–1332 CrossRef CAS.
- L. Shang, L. Zhang and S. Dong, Analyst, 2009, 134, 107–113 RSC.
- K. Kavallieratos, A. J. Sabucedo, A. T. Pau and J. M. Rodriguez, J. Am. Soc. Mass Spectrom., 2005, 16, 1377–1383 CrossRef CAS.
- J. S. Mendy, M. L. Pilate, T. Horne, V. W. Day and M. A. Hossain, Chem. Commun., 2010, 46, 6084–6086 RSC.
- M. A. Hossain, M. Işıklan, A. Pramanik, M. A. Saeed and F. R. Fronczek, Cryst. Growth Des., 2012, 12, 567–571 CAS.
- R. B. Cole and J. Zhu, Rapid Commun. Mass Spectrom., 1999, 13, 607–611 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, J. Chem. Phys., 2006, 125, 194101 CrossRef.
- D. G. Gusev, Organometallics, 2013, 32, 4239–4243 CrossRef CAS.
- C. Huang, R. K. Wang, B. M. Wong, D. J. McGee, F. Léonard, Y. J. Kim, K. F. Johnson, M. S. Arnold, M. A. Eriksson and P. Gopalan, ACS Nano, 2011, 5, 7767–7774 CrossRef CAS.
- B. M. Wong, M. Piacenza and F. D. Sala, Phys. Chem. Chem. Phys., 2009, 11, 4498–4508 RSC.
- B. M. Wong and T. H. Hsieh, J. Chem. Theory Comput., 2010, 6, 3704–3712 CrossRef CAS.
- B. M. Wong and J. G. Cordaro, J. Chem. Phys., 2008, 129, 214703 CrossRef.
- M. E. Foster and B. M. Wong, J. Chem. Theory Comput., 2012, 8, 2682–2687 CrossRef CAS.
- R. M. Richard and J. M. Herbert, J. Chem. Theory Comput., 2011, 7, 1296–1306 CrossRef CAS.
- N. Kuritz, T. Stein, R. Baer and L. Kronik, J. Chem. Theory Comput., 2011, 7, 2408–2415 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, M. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian, Inc., Wallingford CT, 2009.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization of the receptor and additional Job plots. See DOI: 10.1039/c5ra02372f |
| This journal is © The Royal Society of Chemistry 2015 |