Cupreine grafted onto silica as an enantioselective and recyclable catalyst for the 1,4-addition of malonate to trans-β-nitrostyrene†
I. Billault*,
R. Launez and
M.-C. Scherrmann
Université Paris Sud, ICMMO, UMR CNRS 8182, Bâtiment 420, 91405 Orsay Cedex, France. E-mail: isabelle.billault@u-psud.fr
First published on 19th March 2015
Abstract
Preparation of silica supported cupreine (CPN) and its high catalytic performance for the asymmetric 1,4-addition of dimethyl malonate to trans-β-nitrostyrene in some biomass-derived solvents (2-methyl THF, EtOAc and EtOH) are reported for the first time.
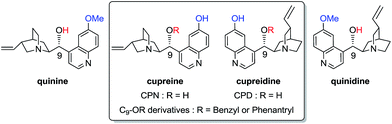
Ten years ago, the pioneering work of Deng and co-workers on Cinchona alkaloids bearing a 6′-hydroxyquinoleine such as cupreine (CPN) or cupreidine (CPD) and their C9–OR derivatives revealed their great efficiency for the 1,4-addition of malonate to nitroalkenes with higher enantioselectivity than their parents quinine or quinidine, respectively (Fig. 1).1,2
Afterward cupreine, cupreidine and their C9–OR derivatives were included in the bifunctional catalyst class and proved their powerful efficiency by catalysing with high enantioselectivity and/or diastereoelectivity different conjugate additions between a large panel of partners3 and other addition reactions (e.g. Friedel–Craft,4 Henry5 and nitroaldol reactions6) together with Kornblum DelaMare rearrangement.7 Recently, Chauhan and Chimni have reviewed all the asymmetric transformations catalysed by the bifunctional catalysts bearing an aromatic hydroxyl group including CPN and its C9 derivatives and proved definitively the interest given to this significant class of catalysts.8
However, all the catalysts reported above have been used only under homogeneous conditions. Unfortunately, such conditions suffer from tedious work-up procedure with tricky separation of catalyst from final product leading to catalyst loss and high cost process. Organocatalyst immobilisation may overcome these limitations by allowing easier recovering of the catalyst and it's recycling. This strategy can lead to a practical and economic catalytic system since higher turnover number (TON) could be reached. Furthermore, grafted catalyst offers the opportunity to implement the catalysis under continuous flow conditions.9
Several Cinchona-based catalysts have been already grafted onto different homemade10 or commercially available polymers.11 Only some examples were reported concerning the grafting of Cinchona alkaloids as organocatalysts onto different porous inorganic supports such as silica (silica gel 60,12 SBA-15,13 MCM-41,12a,13a and Aerosil 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14), Fe3O4 magnetic nanoparticles,15 gold nanoparticles,16 and porous zirconium phosphonate.17
14), Fe3O4 magnetic nanoparticles,15 gold nanoparticles,16 and porous zirconium phosphonate.17
Depending on the Cinchona alkaloid catalyst and the support used, the enantioselectivity performance are mostly maintained or slightly lower compared to their homogeneous counterpart that pointed out the good behaviour of those catalysts for the immobilisation strategy. However, in general the turnover frequency (TOF) decreases for grafted catalyst due to mass transfer resistance and in addition, recycling remains often a challenge. To the best of our knowledge any Cinchona alkaloid bearing a 6′-hydroxyquinoline function has not been anchored onto insoluble support yet. Among all the insoluble supports already used, those based on silica have retained our attention especially for the versatility that their offer to anchor a catalyst: grafting onto preformed silica support ordered or not, as well as by using a co-condensation process. Furthermore, as residual silanol groups (Si–OH) on the silica surface could be not inert during the catalysis, a possible end-capping of those residuals Si–OH is still possible to modify the surface of this support.
Herein we report (i) the first preparation of supported CPN onto the simplest and cheapest amorphous silica support, the silica gel Si 60 Å (70–200 μm), (ii) the catalytic performances of the anchored CPN bifunctional catalyst for the asymmetric 1,4-addition of dimethyl malonate to the trans-β-nitrostyrene especially in solvents derived from biomass as our interest is the development of new process using alternative solvents,18 and (iii) the recycling of the supported catalysts.
CPN was readily prepared in one step from commercially available quinine1 then grafted to the silica support through a thioether bond involving the 3-mercaptopropyl triethoxysilane (MPTES) as a linker between the catalyst and silica.19 Based on this strategy, three alternative routes were tested to prepare different batches of Si-CPN (Scheme 1); route A: the silica surface was first functionalized by mercapto groups (Si–SH), and then CPN was bound through a thiol–ene reaction performed under heterogeneous conditions using 2,2′-azobis(2-methylpropionitrile) (AIBN) as radical initiator; route B involved the end-capping of the residual silanols remaining free at the surface of the silica by using N,N-dimethyl trimethylsilylamine (TMS-DMA)20 before the immobilisation of CPN; finally, route C: the thiol–ene reaction was first carried out under homogeneous conditions to link MPTES to CPN,21 then the adduct (MPTES–CPN) was tethered onto the silica surface after a washing step to remove unreacted MPTES.
All batches of Si-CPN A, B and C were characterized by classical techniques: elemental analysis (C, N and S%), thermogravimetry, BET and FT-IR analysis (See ESI†). Elemental analysis proved a CPN loading of 0.364, 0.268 and 0.225 mmol g−1 of silica for Si-CPN A, B and C, respectively. Thermogravimetric analysis performed on all Si-CPN, patently displayed two different species grafted onto silica surface: a first weight loss was observed at 300 °C for the linker with SH group remaining free, while a second weight loss, consequent to the degradation of the grafted catalyst, was seen at 500 °C. By comparing the N and S% from the elemental analysis, the residual SH groups remaining onto the silica surface after grafting CPN could be estimated too. The residual SH groups were most abundant for Si-CPN A (60%) and B (69%) compare to Si-CPN C (18%). Thereby, the route C offers the possibility to remove most of the residual SH groups onto the silica surface.
The asymmetric Michael addition to nitroalkene is a useful tool for the preparation of versatile optically active building blocks as intermediate in the synthesis of some biological active compounds.1,22 The catalytic performances of supported catalysts Si-CPN A to C were thus evaluated for the asymmetric 1,4 addition of dimethyl malonate to trans-β-nitrostyrene as a model reaction and compared with those obtained with the homogeneous counterpart. Reactions were carried out with trans-β-nitrostyrene 1 (0.5 M) and dimethyl malonate 2 (1.5 eq.) at room temperature in the presence of supported catalysts Si-CPN or CPN under agitation in biomass-derived solvents (Me-THF, AcOEt, EtOH) and in THF for comparison. All results are summarised in Table 1.
| Entry | Catalyst (mol%) | Solvent | Time (h) | Yielda (%) (conv.b (%)) | eec (%) 3 | TOFd |
|---|---|---|---|---|---|---|
| a Isolated yield.b Conv. (%) determined on crude reaction mixture by HPLC with Si-CPN or RMN-1H with CPN.c Determined by HPLC analysis on a Kromasil 3-CelluCoat RP column.d Turn over frequency (mol of 3 per mol per cat per h) calculated using conversion. | ||||||
| 1 | CPN (10) | THF | 24 | 88 (100) | 90 | 0.42 |
| 2 | Si-CPN A (12) | THF | 62 | 90 (95) | 87 | 0.13 |
| 3 | Si-CPN B (27) | THF | 28 | 97 (99) | 73 | 0.13 |
| 4 | Si-CPN C (22) | THF | 72 | 95 (99) | 80 | 0.06 |
| 5 | CPN (10) | MeTHF | 16 | 89 (100) | 92 | 0.62 |
| 6 | Si-CPN A (12) | MeTHF | 41 | 89 (97) | 87 | 0.20 |
| 7 | Si-CPN B (27) | MeTHF | 20 | 90 (94) | 77 | 0.17 |
| 8 | Si-CPN C (22) | MeTHF | 20 | 95 (99) | 88 | 0.22 |
| 9 | CPN (10) | EtOAc | 48 | 84 (100) | 89 | 0.21 |
| 10 | Si-CPN A (12) | EtOAc | 62 | 90 (95) | 82 | 0.13 |
| 11 | Si-CPN B (27) | EtOAc | 48 | 93 (97) | 76 | 0.07 |
| 12 | Si-CPN C (22) | EtOAc | 72 | 93 (97) | 83 | 0.06 |
| 13 | CPN (10) | EtOH | 24 | 97 (100) | 57 | 0.42 |
| 14 | Si-CPN A (12) | EtOH | 30 | 93 (96) | 60 | 0.26 |
| 15 | Si-CPN B (27) | EtOH | 21 | 93 (96) | 42 | 0.17 |
| 16 | Si-CPN C (22) | EtOH | 48 | 92 (98) | 54 | 0.09 |
We were pleased to observe that the Michael addition took place with Si-CPN A (12 mol%) in THF while the reaction did not proceed during the same time in the presence of unmodified silica alone. Furthermore, the ee% of the Michael adduct 3 (S configured) was almost preserved (entries 1 and 2).1 To the best of our knowledge, no conformational analysis of CPN has been investigated yet. Nevertheless, extended conformational studies of the Cinchona alkaloids such as quinine (Fig. 1) have been well explored by NMR in different solvents and calculation approaches. All these analysis have proved that quinine adopts preferentially the so-called anti-open conformation in solution.23 Based on the hypothesis that CPN adopts the same open conformation; three possible models for the arrangement of both reagents 1 and 2 with the catalyst CPN are shown in Fig. 2.
 | ||
| Fig. 2 Postulated models for the 1,4-addition of dimethyl malonate 2 to trans-β-nitrostyrene 1 in the presence of CPN. | ||
In model A we postulated that the π-stacking interaction between the π-donor quinoline ring and the π-acceptor trans-β-nitrostyrene determines the absolute configuration S of product 3 since only the Si face of the double bond of 1 remains accessible for the nucleophile attack of 2. In contrast, both models B and C show a loss of H-bonding with loss of π-stacking interaction or a severe steric interaction, respectively.
As expected, the anchored CPN displayed same stereoselectivity and a TOF only 2.5 times lower compared to its homogeneous counterpart. As the TOF was lower with grafted catalyst Si-CPN A, the 1,4 addition using catalysts Si-CPN B and C were performed with 27 and 22 mol% catalyst, respectively. Unfortunately, with the two latter supported catalysts the ee% were slightly lower in THF, especially when the capped Si-CPN B was used (entries 3 and 4). This last observation was in accordance with results already reported for quinidine grafted onto capped Kieselgel 60.12a,d Moreover, while the TOF of Si-CPN A and B were identical, that of Si-CPN C was halved.
Next, we investigated the Si-CPN catalytic activity by performing the Michael addition in different biomass-derived solvents. MeTHF and AcOEt are not classically used for Michael addition however, as shown in Table 1, both solvents are suitable for this reaction since 92 and 89 ee% were obtained respectively for 3 under homogeneous conditions (entries 5 and 9). Furthermore, we were delighted to observe a better TOF in MeTHF than in THF. Surprisingly the reaction was twice as long as in THF when AcOEt was used as the solvent, leading to a quite poor TOF even under homogeneous conditions (entry 9). By using Si-CPN A or C in MeTHF, the ee% were still comparable to the ones obtained with homogeneous counterpart CPN, while the ee% was always lower with the capped Si-CPN B (entries 5–8). In the presence of AcOEt, all batches of Si-CPN showed a lower catalytic efficiency compared to CPN (entries 9–12). EtOH proved to be not suitable for this reaction since the ee% of 3 decreased drastically whatever the conditions (entries 13–16). In this solvent we noticed that the Michael addition somewhat occurred at 10% conversion without catalyst. Furthermore, we assumed that in a solvent with a strong hydrogen bond donor property such as EtOH, the arrangement between the catalyst and the reactants, based on π-stacking and hydrogen bonding,24 is not as favoured than in an aprotic solvent giving rise to a lower enantioselectivity of the addition (Fig. 2). MeTHF was thus found to be the best biomass-derived solvent (ee% and TOF) giving better results than THF for the asymmetric 1,4-addition of dimethyl malonate to the trans-β-nitrostyrene using silica supported CPN. THF and MeTHF displayed quite similar proprieties excepting that unlike THF, MeTHF is not miscible to water,25 a strong hydrogen bond donor solvent. Thus, MeTHF could probably not desorb the water molecules always adsorbed onto the silica surface leading to water content lower in reaction mixture. As MeTHF offered the best catalytic activities, it was selected to examine the recyclability of the supported Si-CPN (Table 2). The catalysts Si-CPN were recovered by filtration after the reaction, washed then dried under vacuum before the next run.
| Entry | Run | Catalyst (mol%) Time (h) | Conv.b (%) | Yieldc (%) | eed (%) | TONe |
|---|---|---|---|---|---|---|
| a All reactions were carried out in same conditions than for results in Table 1.b Conversion determined by HPLC analysis.c Isolated yield, nd: not determined.d Determined by HPLC analysis on a Kromasil 3-CelluCoat RP column.e Turn over number (mol of 3 per mol per cat) calculated using conversion. | ||||||
| 1 | 1 | Si-CPN A (36%) 16 h | 100 | 95 | 86 | 2.8 |
| 2 | 2 | 99 | 92 | 86 | 5.6 | |
| 3 | 3 | 99 | 96 | 87 | 8.4 | |
| 4 | 4 | 99 | 96 | 84 | 11.2 | |
| 5 | 5 | 97 | 94 | 85 | 13.9 | |
| 6 | 6 | 93 | 90 | 83 | 16.2 | |
| 7 | 7 | 65 | nd | 83 | — | |
| 8 | 1 | Si-CPN B (21%) 32 h | 93 | 89 | 83 | 4.4 |
| 9 | 2 | 94 | 93 | 86 | 8.9 | |
| 10 | 3 | 82 | 77 | 85 | 12.8 | |
| 11 | 4 | 64 | nd | 84 | — | |
| 12 | 1 | Si-CPN C (22%) 20 h | 99 | 95 | 88 | 4.4 |
| 13 | 2 | 97 | 95 | 87 | 8.7 | |
| 14 | 3 | 95 | 91 | 87 | 12.9 | |
| 15 | 4 | 43 | nd | 79 | — | |
All recovered catalysts Si-CPN A to C were able to be recycled at least three and even up to five runs without any loss of catalytic activities (Table 2). As the number of recycling could be dependent of the initial catalyst mol% engaged in the first run, the turn over number (TON) have to be considered to compare them. Thereby, thanks to recycling, all the catalysts Si-CPN nearly exhibited similar TON values around 14.
In conclusion, we have developed a novel heterogeneous chiral catalyst by grafting CPN onto amorphous silica. Our results attested for the first time that silica is a suitable support to immobilise CPN while retaining the asymmetric catalytic performance of this bifuctional catalyst almost unchanged. Among all supported catalysts Si-CPN prepared, the Si-CPN A displayed the best catalytic efficiency despite the presence of residual SH groups onto the silica surface. We are now investigating the use of Si-CPN A for various applications under continuous flow conditions using a packed bed reactor.
Notes and references
- H. Li, Y. Wang, L. Tang and L. Deng, J. Am. Chem. Soc., 2004, 126, 9906–9907 CrossRef CAS PubMed.
- T. Marcelli, J. H. van Maarseveen and H. Hiemstra, Angew. Chem., Int. Ed., 2006, 45, 7496–7504 CrossRef CAS PubMed.
- (a) H. Li, J. Song, X. Liu and L. Deng, J. Am. Chem. Soc., 2005, 127, 8948–8949 CrossRef CAS PubMed; (b) H. Li, Y. Wang, L. Tang, F. Wu, X. Liu, C. Guo, B. M. Foxman and L. Deng, Angew. Chem., Int. Ed., 2005, 44, 105–108 CrossRef CAS PubMed; (c) F. Wu, R. Hong, J. Khan, X. Liu and L. Deng, Angew. Chem., Int. Ed., 2006, 45, 4301–4305 CrossRef CAS PubMed; (d) F. Wu, H. Li, R. Hong and L. Deng, Angew. Chem., Int. Ed., 2006, 45, 947–950 CrossRef CAS PubMed; (e) J. Wang, H. Li, L. Zu and W. Wang, Org. Lett., 2006, 8, 1391–1394 CrossRef CAS PubMed; (f) J. Wang, H. Li, L. Zu, W. Jiang and W. Wang, Adv. Synth. Catal., 2006, 348, 2047–2050 CrossRef CAS; (g) M. Shi, Z.-Y. Lei, M.-X. Zhao and J.-W. Shi, Tetrahedron Lett., 2007, 48, 5743–5746 CrossRef CAS PubMed; (h) F.-X. Chen, C. Shao, Q. Wang, P. Gong, D.-Y. Zhang, B.-Z. Zhang and R. Wang, Tetrahedron Lett., 2007, 48, 8456–8459 CrossRef CAS PubMed; (i) R. Dodda, J. J. Goldman, T. Mandal, C.-G. Zhao, G. A. Broker and E. R. T. Tiekink, Adv. Synth. Catal., 2008, 350, 537–541 CrossRef CAS PubMed; (j) Y.-N. Xuan, S.-Z. Nie, L.-T. Dong, J.-M. Zhang and M. Yan, Org. Lett., 2009, 11, 1583–1586 CrossRef CAS PubMed; (k) W.-B. Chen, Z.-J. Wu, Q.-L. Pei, L.-F. Cun, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2010, 12, 3132–3135 CrossRef CAS PubMed; (l) W. Raimondi, G. Lettieri, J.-P. Dulcère, D. Bonne and J. Rodriguez, Chem. Commun., 2010, 46, 7247–7249 RSC; (m) F. Li, Y.-Z. Li, Z.-S. Jia, M.-H. Xu, P. Tian and G.-Q. Lin, Tetrahedron, 2011, 67, 10186–10194 CrossRef CAS PubMed; (n) P. He, X. Liu, J. Shi, L. Lin and X. Feng, Org. Lett., 2011, 13, 936–939 CrossRef CAS PubMed; (o) H.-F. Wang, H. Xiao, X.-W. Wang and G. Zhao, Tetrahedron, 2011, 67, 5389–5394 CrossRef CAS PubMed; (p) P. Chauhan and S. S. Chimni, Adv. Synth. Catal., 2011, 353, 3203–3212 CrossRef CAS; (q) Y.-Y. Yan, R.-J. Lu, J.-J. Wang, Y.-N. Xuan and M. Yan, Tetrahedron, 2012, 68, 6123–6130 CrossRef CAS PubMed.
- (a) J. Deng, S. Zhang, P. Ding, H. Jinag, W. Wang and J. Li, Adv. Synth. Catal., 2010, 352, 833–838 CrossRef CAS; (b) P. Chauhan and S. S. Chimni, Eur. J. Org. Chem., 2011, 1636–1640 CrossRef CAS; (c) X.-H. Xu, A. Kusuda, E. Tokunaga and N. Shibata, Green Chem., 2011, 13, 46–50 RSC.
- M. Bandini, R. Sinisi and A. Umani-Ronchi, Chem. Commun., 2008, 4360–4362 RSC.
- (a) H. Li, B. Wang and L. Deng, J. Am. Chem. Soc., 2006, 128, 732–733 CrossRef CAS PubMed; (b) T. Mandal, S. Samanta and C.-G. Zhao, Org. Lett., 2007, 9, 943–945 CrossRef CAS PubMed.
- S. T. Staben, X. Linghu and F. D. Toste, J. Am. Chem. Soc., 2006, 126, 12658–12659 CrossRef PubMed.
- P. Chauhan and S. S. Chimni, RSC Adv., 2012, 2, 737–758 RSC.
- D. Zhao and K. Ding, ACS Catal., 2013, 3, 928–944 CrossRef CAS.
- (a) V. Athawale and N. Manjrekar, Tetrahedron Lett., 2001, 42, 4541–4543 CrossRef CAS; (b) K. Onimura, K. Matsuzaki, Y. K. Lee, H. Tsutsumi and T. Oishi, Polym. J., 2004, 36, 190–197 CrossRef CAS; (c) S. H. Youk, S. H. Oh, H. S. Rho, J. E. Lee, J. W. Lee and C. E. Song, Chem. Commun., 2009, 2220–2222 RSC; (d) K. A. Fredriksen, T. E. Kristensen and T. Hansen, Beilstein J. Org. Chem., 2012, 8, 1126–1133 CrossRef CAS PubMed; (e) D. Cantoni, A. Mandoli, R. P. Jumbe and D. Pini, Eur. J. Org. Chem., 2012, 1336–1345 Search PubMed.
- (a) R. Alvarez, M.-A. Ourdin, C. Cavé, J. d'Angelo and P. Chaminade, Tetrahedron Lett., 1999, 40, 7091–7094 CrossRef CAS; (b) F. Bonfils, I. Cazaux, P. Hodge and C. Caze, Org. Biomol. Chem., 2006, 4, 493–497 RSC; (c) Y. Qin, G. Yang, L. Yang, J. Li and Y. Cui, Catal. Lett., 2011, 141, 481–488 CrossRef CAS; (d) R. P. Jumbe, A. Mandoli, F. De Lorenzi, D. Pini and P. Salvadori, Adv. Synth. Catal., 2010, 352, 1434–1440 CrossRef; (e) L. Yang, D. Zhou, C. Qu and Y. Cui, Catal. Lett., 2012, 142, 1405–1410 CrossRef CAS; (f) Y. Qin, W. Zhao, L. Yang, X. Zhang and Y. Cui, Chirality, 2012, 24, 640–645 CrossRef CAS PubMed.
- (a) F. Bigi, S. Carloni, R. Maggi, A. Mazzacani, G. Sartori and G. Tanzi, J. Mol. Catal. A: Chem., 2002, 182–183, 533–539 CrossRef CAS; (b) H. S. Kim, Y.-M. Song, J. S. Choi, J. W. Yang and H. Han, Tetrahedron, 2004, 60, 12051–12057 CrossRef CAS PubMed; (c) W. Zhao, Y. Zhang, C. Qu, L. Zhang and Y. Cui, Catal. Lett., 2014, 144, 1681–1688 CrossRef CAS PubMed; (d) S. H. Newland, D. J. Xuereb, E. Gianotti, L. Marchese, R. Rios and R. Raja, Catal. Sci. Technol., 2015, 5, 660–665 RSC.
- (a) P. Yu, J. He and C. Guo, Chem. Commun., 2008, 2355–2357 RSC; (b) Q. Chen, C. Xin, L.-L. Lou, K. Yu, F. Ding and S. Liu, Catal. Lett., 2011, 141, 1378–1383 CrossRef CAS.
- (a) J. Hong, I. Lee and F. Zeara, Top. Catal., 2011, 54, 1340–1347 CrossRef CAS; (b) J. Hong and F. Zaera, J. Am. Chem. Soc., 2012, 134, 13056–13065 CrossRef CAS PubMed.
- O. Gleeson, G.-L. Davies, A. Peschiulli, R. Tekoriute, Y. K. Gun'ko and S. J. Connon, Org. Biomol. Chem., 2011, 9, 7929–7940 CAS.
- L. Santacruz, S. Niembo, A. Santillana, A. Shafir and A. Vallribera, New J. Chem., 2014, 38, 636–640 RSC.
- W. Wang, X. Ma, J. Wan, J. Cao and Q. Tang, Dalton Trans., 2012, 41, 5715–5726 RSC.
- (a) N. Prosa, R. Turgis, R. Piccardi and M.-C. Scherrmann, Eur. J. Org. Chem., 2012, 2188–2200 CrossRef CAS; (b) R. Turgis, I. Billault, S. Acherar, J. Augé and M.-C. Scherrmann, Green Chem., 2013, 15, 1016–1029 RSC; (c) I. Billault, F. Pessel, A. Petit, R. Turgis and M.-C. Scherrmann, New J. Chem., 2015, 39, 1986–1995 RSC.
- A. Mandl, L. Nicoletti, M. Lämmerhofer and W. Lindner, J. Chromatogr. A, 1999, 858, 1–11 CrossRef CAS.
- L. L. Crowe and L. M. Tolbert, Langmuir, 2008, 24, 8541–8546 CrossRef CAS PubMed.
- A. K. Tucker-Schwartz, R. A. Farrell and R. L. Garell, J. Am. Chem. Soc., 2011, 133, 11026–11029 CrossRef CAS PubMed.
- (a) O. M. Berner, L. Tedeschi and D. Enders, Eur. J. Org. Chem., 2002, 1877–1894 CrossRef CAS; (b) T. Okino, Y. Hoashi, T. Furukawa, X. Xu and Y. Takemoto, J. Am. Chem. Soc., 2005, 127, 119–125 CrossRef CAS PubMed.
- (a) G. D. H. Dijkstra, R. M. Kellogg and H. Wynberg, Recl. Trav. Chim. Pays-Bas, 1989, 108, 195–204 CrossRef CAS; (b) G. D. H. Dijkstra, R. M. Kellogg, H. Wynberg, J. S. Svendsen, I. Marko and K. B. Sharpless, J. Am. Chem. Soc., 1989, 111, 8069–8076 CrossRef CAS; (c) G. D. H. Dijkstra, R. M. Kellogg and H. Wynberg, J. Org. Chem., 1990, 55, 6121–6131 CrossRef CAS.
- Y. Sohtome and K. Nagasawa, Chem. Commun., 2012, 48, 7777–7789 RSC.
- D. F. Aycock, Org. Process Res. Dev., 2007, 11, 156–159 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis procedures and characterisation of Si-CPN: elemental analysis, thermogravimetry, BET and FT-IR analysis. See DOI: 10.1039/c5ra02292d |
| This journal is © The Royal Society of Chemistry 2015 |