Preparation and characterization of a new cement-based composite with sulfur polymer†
Seunggun Yu‡
*a,
Hyuk Kwon‡bc,
Hyuk Rae Nohb,
Bo-In Parkb,
No Kyung Parkb,
Heon-Jin Choia,
Sung-Churl Choic and
Goo Dae Kim*b
aDepartment of Materials Science and Engineering, Yonsei University, Seoul, 120-749, Republic of Korea. E-mail: kist@yonsei.ac.kr
bInterface Control Research Center, Korea Institute of Science and Technology, Hwarangro 14-gil 5, Seongbuk-gu, Seoul 136-791, Republic of Korea
cDivision of Materials Science and Engineering, Hanyang University, 222 Wangsimni-ro, Seongdong-gu, Seoul 133-791, Republic of Korea
First published on 15th April 2015
Abstract
The addition of sulfur polymer (SP) and the resulting effects on the hydration and physical properties of polymer-cement mortars were investigated. Sulfur polymer-cement mortars (SPMs) with 5 wt%, 10 wt%, and 20 wt% of SPs were prepared to evaluate the compressive strength and impermeability in comparison to ordinary Portland cement mortars (PCMs). After curing for 28 days, the compressive strength of SPM with 5 wt% of SP increased by approximately 8.0% compared to PCM by hardened SP binder as well as proper hydration of hydrates. The impermeability of SPMs cured for 28 days was two times higher than that of PCM cured for the same period. The outstanding enhancement of the compressive strength and impermeability are attributed to SP additive formed inside the hydrated cement.
Introduction
Polymer modifiers have received significant attention to complement the lack of traditional Portland cement, which is easily deteriorated by acidic rain, saline deicing agents, and freeze–thaw processes.1–4 In general, polymer modifiers, which exist in the form of latex, liquid, or powder, emulsify or get dispersed when mixing with cement or water, leading to a protective polymer layer.5,6 Thermoset resins with low molecular weight have been applied to form a co-matrix phase consisting of the thermoset resin and cement. The resin incorporated into cement hydrates strengthens through cross-linking reaction,7 leading to an improvement of the structural strength. The incorporated polymeric phase makes the cement material highly adhesive, impermeable, chemically resistant, and mechanically flexible.8–10For a long time there has been an attempt to use sulfur, which is one of the most abundant elements that is used as construction material, vulcanization agent for synthetic rubber, and precursor for the production of sulfuric acid.11 More than 60 million tons of elemental sulfur has been globally produced from the refining of petroleum products.12,13 However, the use of elemental sulfur has disadvantages such as low flashing temperature (Tf = 207 °C), spontaneous ignition temperature (Ts = 245 °C), and brittleness by crystallographic nature, including orthorhombic, monoclinic, and amorphous phases, the latter forming an eight-membered rings (S8) under ambient temperature and pressure.14,15 To solve these problems, several groups have reported newly modified sulfur polymer (SP) using dicyclopentadiene (DCPD),16 propylene sulfide,17,18 2,2-dimethylthiirane,19 and styrene,20 which can provide stabilized and plasticized sulfur materials via copolymerization with S8. As a result, SP could be used as a binder for aggregate waste recycling and radioactive waste, based on the higher adhesive strength of SP.21–23 The applications of SP have been widened to include building and construction elements, marine structures and plants based on the good chemical resistance, rapid setting time, high strength, high elasticity, high impermeability, and other favorable properties.24–27 However, there are still limitations for buildings and chemical plants application due to their low melting temperature and inflammability, which directly affect accident and human life.
Herein, we report the use of SP as an additive to enhance the physical properties of traditional Portland cement mortar. The sulfur polymer-cement mortar (SPM) was easily prepared by mixing cement, sand, SP, and water at a low temperature of approximately 55 °C, in which the SP maintains its molten state. The addition of SP demonstrated cement mortar with approximately 8.0% increased compressive strength without any structural degradation compared to PCM as well as 67.8% decreased impermeability.
Experimental section
Materials
Sulfur powder (99.9%), pyridine (99.9%), and ethanol (99.5%) were purchased from Samchun Chemical, South Korea. Dicyclopentadiene (DCPD), with an oligomer of more than 80%, was used to inhibit a highly exothermic reaction during polymerization and was purchased from Kolon Industries, South Korea, as shown in Table S1.† The oligomeric DCPD was kept into refrigerator with cool and dry condition to inhibit the polymerization of cyclopentadiene even at ambient condition. Standardized ordinary Portland cement (OPC) was purchased from Hanil Cement, South Korea. The chemical composition and physical properties of the Portland cement are noted in Table S2 and S3,† respectively. Standard sand, stipulated by ISO 679:1989, was purchased from Jumunjin Silica Sand Co., Ltd., South Korea and maintained so as to ensure uniform test conditions and eliminate the effect of varied sand particle size.Copolymerization of sulfur and DCPD
The copolymerization of sulfur and DCPD was performed according to our previous work.28 Briefly, 100 g of sulfur was sealed in a 3-neck flask and heated on a hot plate to 140 °C under vigorous stirring. Then, 30 mL of DCPD oligomer was added into the molten sulfur and 3.0 mL of pyridine was added. The reaction between DCPD and sulfur was promoted by free-radical chain process at the low temperature of about 140 °C,29,30 while the sulfur molecules were intrinsically polymerized above 159.4 °C.31,32 The 3.0 mL of pyridine, which acts as a co-solvent of the sulfur and DCPD, was added into the liquid mixture of molten sulfur and DCPD to stabilize the exothermic reaction. After completion of the reaction, the mixture became a single-phase solution to be used for the bulk copolymerization process. The mixture was stirred with reflux to inhibit the evaporation of pyridine for 2 hours at 140 °C, and it transformed into a brown viscous mixture.Preparation of SPM
Pristine cement mortar was prepared with a water–cement ratio of 0.5 at room temperature, according to the ISO 679:1989 standard. The SPM was prepared in line with the KS F 2476 standard, which considers a polymer additive in the cement mortar. The cement, sand, and water were mixed at 55 °C for 1 min, and the pre-molten SP was added to the mixture and mixed for a further 4 min by a shear force. The compositions and processing conditions are noted in Table S4.† The mixture was cured in a chamber at 20 °C and 95% humidity for 24 hours. The specimens were further cured underwater at 25 °C and were taken out at the given time of 3, 7, 14 and 28 days. To evaluate the hydration degree, the specific samples were soaked in ethanol for 24 hours to inhibit the hydration progress, followed by drying under vacuum at 60 °C until ethanol was fully removed.Characterization
The thermal decomposition behavior of the SP and the formation behavior of calcium hydroxide were investigated by thermogravimetric analysis (TGA, Q-50, TA Instruments Inc.) under N2 atmosphere with 10 °C min−1 using platinum crucible. The hydration behavior was investigated by X-ray diffraction (XRD, X'pert Pro. PANalytical, Netherlands) and the XRD data were analyzed by the X'Pert HighScore Plus software. The compressive strength was evaluated using a compressive strength machine (FS-1050A, Jeil Precision, South Korea). The morphologies of the SP films in the composites were evaluated using scanning electron microscopy (SEM, S-4100, Hitachi, Japan). To obtain a clear view of the film formation in mortar, mortar specimens were etched with 0.1 N HCl solution for 3 hours to remove inorganic components. After etching, the specimens were washed with distilled water and dried under vacuum for 24 hours at 60 °C. The porosity was measured using mercury intrusion porosimeter (MIP, AutoPore IV 9500 V 1.08, USA). To determine the absorption rate dependence on the curing time, the specimens were cured underwater at 25 °C, and the absorption rates were determined by calculating the change in weight after taking the specimens out of the water after 3, 7 and 28 days, followed by drying under vacuum for 24 hours at 60 °C according to KS F 2451.Results and discussion
Hydration behaviour of SPMs
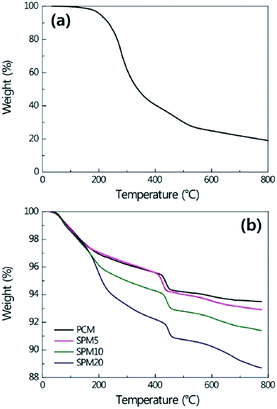
Fig. 1a shows a TGA curve of the SP. The weight rapidly decreased above 200 °C because the backbone of the SP containing aromatic rings was carbonized, resulting in a residual weight of 20 wt% at 800 °C. Fig. 1b shows the TGA curves of the SPMs cured for 28 days. In the case of PCM, the weight decrease at 100–200 °C and 400–460 °C was caused by mass loss attributed to the chemically combined water in cement hydrates and the dehydration of Ca(OH)2, respectively. Above 500 °C, the weight loss came from the decarboxylation of CaCO3 with a consideration of weight loss from SP. The dehydration of calcium hydroxide in SPM could be quantified from the weight loss between 400 and 460 °C, by the following equation, as suggested by Taylor.33| WCa(OH)2 = WLCa(OH)2 × (MWCa(OH)2/MWH2O) |
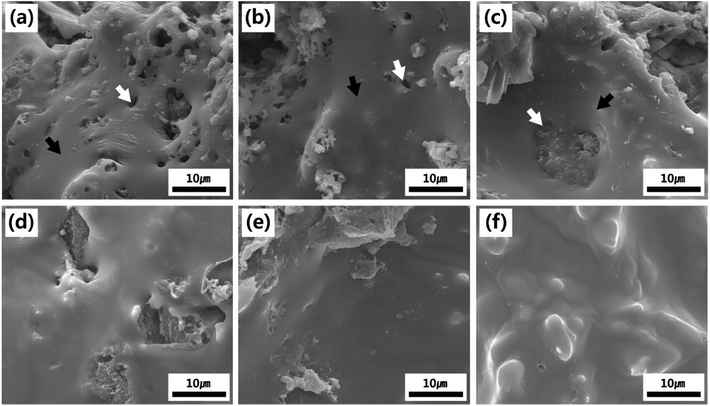
Fig. 2a–d shows the morphological hydration behavior of PCM, SPM5, SPM10 and SPM20 cured for 1 day, respectively. The PCM cured for 1 day shows a few calcium hydroxides hydrated from calcium silicate as shown in Fig. 2a. Meanwhile, the SPMs cured for 1 day dominantly shows ettringite crystals (long rod-shaped hydrates) surrounded with SP additive, unlike that of PCM, as shown in Fig. 2b–d. Moreover, the ettringite crystals were grown by 3 μm in length with an increase in the content of SP. It is ascribed that the sulfate ion from partially dissolved SP in the alkaline environment by cement accelerates the formation and growth of ettringite at the early stage of curing. Also, the fibrous ettringite crystals enable the improvement of initial strength. After 7 days of curing time, not only densification of hydrates in the all samples was undertaken, but also the ettringite crystals in SPMs clearly disappeared through reaction with tricalcium aluminate (3CaO·Al2O3), as shown in Fig. S1a–d.† After 28 days of curing time, the hydrates was densely grown as shown in Fig. 2e–h. Especially, the SPM5 shows completely hardened hydrates without any cracks similar to PCM as shown in Fig. 2e and f. Meanwhile, the SPM10 and SPM20 show the partially defective hardened hydrates, which is ascribed that the excess SP disturbed the natural hydration reaction as shown in Fig. 2g and h.
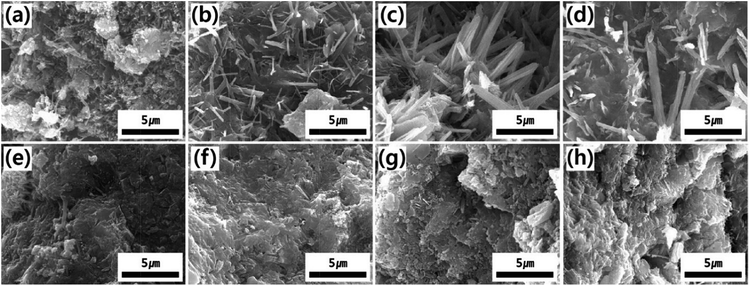 | ||
| Fig. 2 SEM images of (a) PCM, (b) SPM5, (c) SPM10 and (d) SPM20 cured for 1 day, and (e) PCM, (f) SPM5, (g) SPM10 and (h) SPM20 cured for 28 days, respectively. | ||
Fig. 3 shows XRD patterns of PCM and SPMs cured for 28 days. It was shown that both PCM and SPMs were composed of similar hydrate components. The peak from quartz at about 27° is the most intensive by incorporated sand which possess large volume. The peaks from portlandite (P) and calcite (C) were distinct, which were grown during the hydration process without inhibition by SP for hydration. The intensity of the crystalline peak of portlandite at 18°, gradually decreased with an increase in the content of SP, which was similar to the change of the content of Ca(OH)2, as calculated on the basis of the TGA analysis.
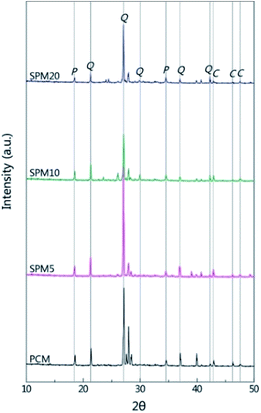 | ||
| Fig. 3 XRD patterns of PCM and SPMs cured for 28 days. The patterns were indexed according to quartz (Q), portlandite (P), and calcite (C). | ||
Pore-size distribution and compressive strength of SPMs
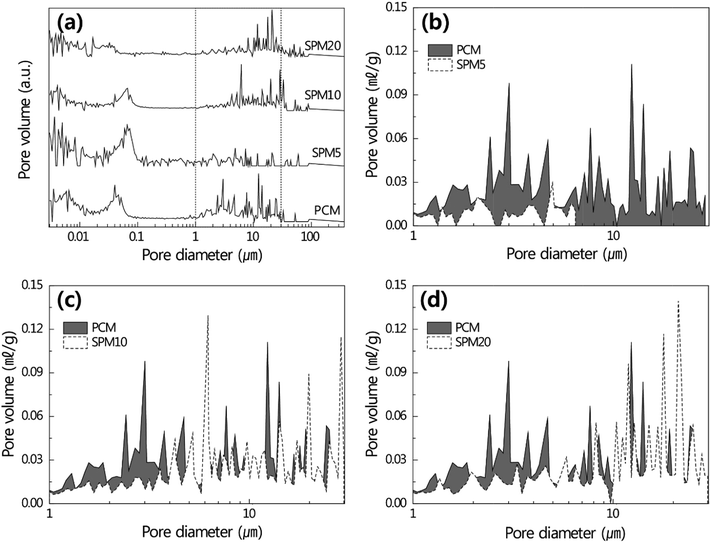
Fig. 4a shows the total pore size distributions of PCM and SPMs cured for 28 days. In the distribution below 1 μm, the pore size increased with an increase in the content of SP, but the pore size distribution of SPM20 decreased quite a lot. This is due to the excess SP disrupted by the formation of sub-micron-sized capillary pore with the progress of hydration reaction. The pore size shows mainly popular distribution over 1 μm, which affects mechanical performance. To further confirm the formation of micron-sized pores, the distribution in the range from 1 μm to 30 μm was magnified as shown in Fig. 4b–d. SPM5 shows the peak shift towards the left, resulting in a reduction of the overall size of the micron-sized pores as shown in Fig. 4b. However, the overall size of micron-sized pores in the SPM10 of the SP increased, exhibiting a similar distribution like PCM as shown in Fig. 4c.Meanwhile, the SPM20 shows the peak shift towards the right, compared to the PCM, resulting in an increased overall size of the micron-sized pores as shown in Fig. 4d. Especially, the SPM5 exhibited the lowest total porosity of approximately 14.0%, while the PCM exhibited the total porosity of approximately 20.1% as shown in Fig. S2.† The mixed SP existed between hydrates in the hardened cement body with a porous structure. The proper amount of SP in the SPM5 filled in micron-sized pores, leading to a smaller pore-size distribution below 10 μm as well as decreased porosity with minimizing disturbance of hydration of cement. Meanwhile, in the SPM10 and SPM20, the hydration process of cement was strongly disturbed by excess SP, and a more porous structure was formed in the hardened cement body. Consequently, the excess SP caused a larger pore-size distribution above 10 μm, even though the SP filled the pores.
Fig. 5 shows the compressive strengths of the PCM and SPMs as a function of curing time. In the whole curing time, the compressive strength of the SPMs decreased with an increase in the content of SP. Before 14 days of curing time, the SPMs had a higher compressive strength than PCM, but after 14 days of curing time, the strength of SPM10 was similar to that of PCM and SPM20 had a slight loss in the strength. Meanwhile, SPM5 exhibited the highest compressive strength in the whole curing time, which was an enhancement of approximately 8% compared to PCM cured for 28 days. Before 14 days of curing time, the increase in the strength of SPMs was caused by hardened SP binders, even although the hydration was disturbed by the SP. After 14 days of curing time, the increase in the strength of SPM5 was synergistically caused by minimizing hydration damage and hardening of SP binder in pores, because a small amount of the SP did not influence the additional hydration reaction. Meanwhile, hydration of SPM10 and SPM20 was suppressed by excess SP, leading to a decrease in the compressive strength.
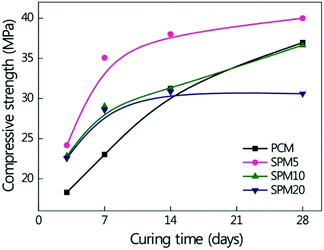 | ||
| Fig. 5 Compressive strength of PCM and SPMs with an increase in the curing time and the content of SP. Full lines are to guide the eye. | ||
Impermeability behaviour of SPMs
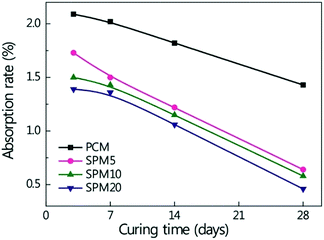
Fig. 6a–c shows SP film formed on the hydrated cements after 3 days of curing time. The SP film in SPM5 was incompletely formed as shown in the Fig. 6a. It is ascribed that pores having a size of 1–5 μm were generated by hydrate residues or the existence of free water in the SP film. The relatively small sized pores disappeared and fully continuous SP film were formed on the hydrated cement with an increase in the content of SP, as shown in the Fig. 6b and c. Fig. 6d–f shows SP film formed in the hydrated cements after 28 days of curing time. The grown SP films had uniform and smooth surface. The pores in the SPMs cured for 3 days disappeared regardless of the content of the SP, because the free water transforms into crystallized water with an increase in the curing time, in which free water caused the porous structure of the SP films. Moreover, the SP had a ‘semi-solid state’ at a room temperature and gradually filled the pores as illustrated in Fig. S3.† A similar process of the formation of polymeric films in cement was previously observed.36,37Fig. 7 shows the water absorption rate of the SPMs and PCM from 3 days to 28 days. The absorption rate decreased with an increase in the content of SP and was smaller than that of PCM in the whole curing time. Furthermore, the absorption rate of SPMs gradually decreased with an increase in the curing time. SPM20 cured for 28 days exhibited the absorption rates decreased by 67.8% from 1.43% to 0.46% compared to that of PCM cured for 28 days. It is ascribed that the SP films were formed inside hardened cement hydrate, and the films solidified with an increase in the curing time. The incorporated SP films existing around cement hydrate prevent penetration of water molecules from outside, because of the hydrophobicity by sulfur in the SP films as well as uniformity of the continuous film.
Conclusions
We successfully synthesized a SP with a low temperature workability to demonstrate a polymeric additive that can be easily mixed with water and cement. Unlike the traditional mixing, the cement, water and sand were mixed with the molten SP in proper heating environment. The size distribution of micron-sized pores became smaller than that of PCM, unlike the SPMs with SP above 10 wt% that show increased micron-sized pore size distribution. Consequentially, the SPM5 showed increased compressive strength compared to SPM10, SPM20, and PCM in the whole curing time. Furthermore, the impermeability was greatly increased due to the hydrophobicity of the SP dispersed inside the hardened cement hydrates. Our approach provides a simple method to produce versatile polymer cement mortar using a novel SP.Acknowledgements
This work was financially supported by the Korea Institute of Science and Technology (KIST), Korea.Notes and references
- D. W. Fowler, Cem. Concr. Compos., 1999, 21, 449–452 CrossRef CAS.
- J. Wang, S. Zhang, H. Yu, X. Kong, X. Wang and Z. Gu, Cem. Concr. Compos., 2005, 27, 920–925 CrossRef CAS PubMed.
- E. Sakai and J. Sugita, Cem. Concr. Compos., 1995, 25, 127–135 CrossRef CAS.
- Y. Ohama and O. Masahiro, Adv. Mater. Res., 2013, 687, 26–34 CrossRef.
- H. B. Wagner, Ind. Eng. Chem. Prod. Res. Dev., 1965, 4, 191–196 CrossRef CAS.
- M. M. Alonso, M. Palacios and F. Puertas, Ind. Eng. Chem. Res., 2013, 52, 17323–17329 CrossRef CAS.
- Y.-W. Mai and B. Cotterell, Cem. Concr. Res., 1986, 16, 646–652 CrossRef CAS.
- E. Knapen and D. Van Gemert, Cem. Concr. Res., 2009, 39, 6–13 CrossRef CAS PubMed.
- M. U. K. Afridi, Y. Ohama, K. Demura and M. Z. Iqbal, Cem. Concr. Res., 2003, 33, 1715–1721 CrossRef CAS.
- N. Ukrainczyk and A. Rogina, Cem. Concr. Compos., 2013, 41, 16–23 CrossRef CAS PubMed.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, S. R. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- G. Kutney, Sulfur: history, technology, applications & industry, ChemTec Publishing, Toronto, 2007 Search PubMed.
- W. J. Chung, A. G. Simmonds, J. J. Girebel, E. T. Kim, H. S. Suh, I.-B. Shim, R. S. Glass, D. A. Loy, P. Theato, T.-E. Sung, K. Char and J. Pyun, Angew. Chem., Int. Ed., 2011, 50, 11409–11412 CrossRef CAS PubMed.
- B. Meyer, Chem. Rev., 1976, 76, 367–388 CrossRef CAS.
- J. J. Griebel, N. A. Nguyen, A. V. Astashkin, R. S. Glass, M. E. Mackay, K. Char and J. Pyun, ACS Macro Lett., 2014, 3, 1258–1261 CrossRef CAS.
- D. J. Bourne, New Uses of Sulfur—II, American Chemical Society, 1978 Search PubMed.
- A. Duda and S. Penczek, Macromolecules, 1982, 15, 36–40 CrossRef CAS.
- S. Penczek, R. Slazak and A. Duda, Nature, 1978, 273, 738–739 CrossRef CAS.
- A. Duda and S. Penczek, Makromol. Chem., 1980, 181, 995–1001 CrossRef CAS PubMed.
- L. B. Blight, B. R. Currell, B. J. Nash, R. T. M. Scott and C. Stillo, Br. Polym. J., 1980, 12, 5–11 CrossRef CAS PubMed.
- A.-M. O. Mohamed and M. M. El-Gamal, J. Hazard. Mater., 2011, 192, 576–584 CrossRef CAS PubMed.
- F. A. López, F. J. Alguacil, O. Rodríguez, M. J. Sierra and R. Millán, Waste Manage., 2015, 35, 301–306 CrossRef PubMed.
- M. Fuhrmann, D. Melamed, P. D. Kalb, J. W. Adams and L. W. Milian, Waste Manage., 2002, 22, 327–333 CrossRef CAS.
- A.-M. O. Mohamed and M. El-Gamal, Cem. Concr. Compos., 2009, 31, 186–194 CrossRef CAS PubMed.
- H. Weber, W. McBee and E. Krabbe, Mater. Perform., 1990, 29, 73–77 CAS.
- A.-M. O. Mohamed and M. El-Gamal, Sulfur concrete for the construction industry: a sustainable development approach, J. Ross Publishing, 2010 Search PubMed.
- W. C. McBee, H. Weber and F. E. Ward, ACI Spec. Publ., 1989, 116, 193–210 Search PubMed.
- G.-D. Kim and T.-B. Kim, J. Ceram. Process. Res., 2007, 8, 82–86 Search PubMed.
- L. Bateman and C. G. Moore, in Organic Sulfur Compounds, ed. N. Kharasch, Pergamon, New York, 1961, vol. 1, ch. 20, pp. 210–228 Search PubMed.
- B. K. Bordoloi, E. M. Pearce, L. Blight, B. R. Currell, R. Merrall, R. A. M. Scott and C. Stillo, J. Polym. Sci., Polym. Chem. Ed., 1980, 18, 383–406 CrossRef CAS PubMed.
- B. Meyer, Chem. Rev., 1976, 76, 367–388 CrossRef CAS.
- A. G. Kalampounias, K. S. Andrikopoulos and S. N. Yannopoulos, J. Chem. Phys., 2003, 118, 8460–8467 CrossRef CAS PubMed.
- G. Alexandre, P. Arnaud and G. Rene, Cem. Concr. Compos., 2006, 28, 12–20 CrossRef PubMed.
- P. Lawrence, M. Cyr and E. Ringot, Cem. Concr. Res., 2003, 33, 1939–1947 CrossRef CAS.
- M. Cyr, P. Lawrence and E. Ringot, Cem. Concr. Res., 2005, 35, 719–730 CAS.
- Y. Ohama, Handbook of polymer-modified mortars and concretes—Properties and process technology, Noyes Publications, Saddle River, 1995 Search PubMed.
- J. Schulze and O. Killermann, Cem. Concr. Res., 2001, 31, 357–362 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM images, schematic illustration, chemical composition and physical properties of Portland cement, experimental variable, and content of dehydrated calcium hydroxide. See DOI: 10.1039/c5ra02281a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |