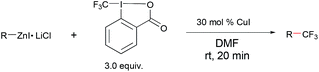
Copper-catalyzed trifluoromethylation of organic zinc reagents with an electrophilic trifluoromethylating reagent†
Chang-Sheng Wanga,
Haoyang Wangb and
Cheng Yao*a
aCollege of Science, Nanjing Tech University, 30 Puzhu South Road, Nanjing 211816, China. E-mail: yaochengnjut@126.com; yaocheng@njtech.edu.cn
bShanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 3rd March 2015
Abstract
A copper-catalyzed trifluoromethylation of aryl, vinyl and alkyl zinc reagents with Togni's reagent was described. Mechanistic studies indicated that the aryl group is initially transferred from the zinc reagent to hypervalent iodine to form a tri-substituted hypervalent iodine intermediate. Consequent reductive-elimination via a concerted bond-forming step and/or radical pathway from this intermediate generates the trifluoromethylated arenes.
It is widely recognized and accepted that the introduction of fluorine into small molecules will bring significant beneficial effects such as improved pharmacokinetics, increased binding selectivity and enhanced metabolic stability.1 Thus, incorporation of fluorine into drug-like molecules has become an indispensible strategy for medicinal chemists in drug design and a daily routine practice for the discovery of new lead compounds.2 Among many fluorinated functional groups, the trifluoromethyl group (CF3–) is highly valuable because it might improve the drug's cellular membrane permeability, and thus its bioavailability.3 As a result, in the past five years, tremendous efforts have been directed towards the development of efficient methods for the formation of C–CF3 bonds. Consequently, significant advances have been achieved in transition metal-catalyzed direct trifluoromethylation of aryl halides, aryl boronic acids or arenes.4,5
In 2011, Shen's group reported a copper-catalyzed trifluoromethylation of aryl boronic acids using Togni's reagent 1 as the trifluoromethyl source.4 The reaction was conducted under mild conditions and was compatible with a variety of functional groups. Inspired by this investigation, we envisaged that an efficient trifluoromethylation method would be developed if a zinc reagent could be employed as the nucleophile since zinc reagent has been demonstrated to be an effective coupling partner in a number of transition metal-catalyzed cross-coupling reactions.6 Herein, we disclose a copper-catalyzed trifluoromethylation of aryl, vinyl and alkyl zinc reagents using Togni's reagent 2 as the trifluoromethyl source. Mechanistic studies indicated that the aryl group from the zinc reagent is initially transferred to hypervalent iodine to form a tri-substituted hypervalent iodine intermediate. Consequent reductive-elimination via a concerted bond-forming step and/or trifluoromethyl radical pathway from this intermediate generates the trifluoromethylated arenes.
We began our study with the reaction of 4-biphenyl zinc reagent 3 with Togni's reagent 1 (ref. 7 and 8) in the presence of a catalytic amount of CuI/1,10-phenanthroline (Phen) in different solvents.
To our surprise, the formation of trifluoromethylated biphenyl was observed in less than 10% yield in solvents such as THF, diethyl ether, diglyme, dioxane, 1,2-dichloroethane or DMF (Table 1, entries 1–6). Interestingly, when Togni's reagent 2 was used, reaction of 4-biphenyl zinc reagent 3 occurred smoothly at room temperature to give the desired product in 13% yield after 12 h at room temperature (Table 1, entry 7). The yield was improved to 42% when the reaction was conducted in the absence of 1,10-phenanthroline (Table 1, entry 8). The effect of the copper salts was then investigated. Nevertheless, the yield of the desired product did not increased significantly when other copper salts such as CuCN, CuCl, CuBr, Cu(MeCN)4PF6 or Cu(OAc)2 were used (Table 1, entries 9–13). Since it was observed that side products such as trifluoromethane and iodotrifluoromethane were formed in the reaction mixture, it is likely only part of the Togni's reagent was converted to the final product. We then studied the effect of the amount of the Togni's reagent. The yield was improved to 50% and 61%, respectively, when 2.0 or 3.0 equivalents of reagent 2 was used (Table 1, entries 14–15). Furthermore, when 30 mol% of CuI was used, the yield was further increased to 90% (Table 1, entry 17). Carefully monitoring of the reaction showed that the reaction was completed within 20 min at room temperature (Table 1, entry 18). Higher temperature was detrimental to the reaction. The yield dropped to 65% when the reaction was conducted at 40 °C (Table 1, entry 20).
| Entry | CF3 reagent | CuX | Ligand | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-biphenyl zinc reagent (0.1 mmol), Togni's reagent (0.1 mmol), CuX (10 mol%), ligand (20 mol%), solvent (1.0 mL) at room temperature for 12 h.b Yields were determined by 19F NMR analysis of the crude product using p-fluorotoluene as an internal standard.c 2.0 equivalents of reagent 2 was used.d 3.0 equivalents of reagent 2 was used.e 3.0 equivalents of reagent 2 and 20 mol% of CuI were used.f 3.0 equivalents of reagent 2 and 30 mol% of CuI were used.g Reaction was conducted for 20 min; isolated in parentheses.h Reaction was conducted at 0 °C.i Reaction was conducted at 40 °C. | |||||
| 1 | 1 | Cul | Phen | THF | — |
| 2 | 1 | Cul | Phen | Et2O | — |
| 3 | 1 | Cul | Phen | Diglyme | — |
| 4 | 1 | Cul | Phen | 1,4-Dioxane | — |
| 5 | 1 | Cul | Phen | ClCH2CH2Cl | — |
| 6 | 1 | Cul | Phen | DMF | — |
| 7 | 2 | Cul | Phen | DMF | 13 |
| 8 | 2 | Cul | — | DMF | 42 |
| 9 | 2 | CuCN | — | DMF | 42 |
| 10 | 2 | CuCl | — | DMF | 24 |
| 11 | 2 | CuBr | — | DMF | 36 |
| 12 | 2 | Cu(MeCN)4PF6 | — | DMF | 35 |
| 13 | 2 | Cu(OAc)2 | — | DMF | — |
| 14 | 2 | Cul | — | DMF | 50c |
| 15 | 2 | Cul | — | DMF | 61d |
| 16 | 2 | Cul | — | DMF | 72e |
| 17 | 2 | Cul | — | DMF | 90f |
| 18 | 2 | Cul | — | DMF | 90g (64) |
| 19 | 2 | Cul | — | DMF | 90h |
| 20 | 2 | Cul | — | DMF | 65i |
With a relied procedure in hand, we then examined the scope of the copper-catalyzed trifluoromethylation of the zinc reagents, and the results were summarized in Table 2. In general, reactions of both electron-rich and electron-poor aryl zinc reagents occurred in good yields (Table 2, 4a–f). Heteroaryl zinc reagents such as pyridyl and quinolinyl zinc reagents also reacted under the optimized conditions to give the corresponding trifluoromethylated heteroarenes in good yields (Table 2, 4g–i). Likewise, reaction of vinyl zinc reagent 3j also afforded the trifluoromethylated alkene in 57% yield (Table 2 and 4j). Similarly, alkyl zinc reagents could also be trifluoromethylated under the optimized reaction conditions (Table 1, entry 4k–m). One advantage of using zinc reagents is its tolerance of functional groups. As illustrated in Table 2, various functional groups such as ester, cyano, protected ketone and amide were compatible.
Typically, reaction of Togni's reagent 2 was proposed to proceed via a pathway involving an in situ generated CF3 radical species.8 To probe if the copper-catalyzed trifluoromethylation of zinc reagents proceeds via a radical mechanism, we conducted two sets of different experiments. In the first experiment, 1.5 equivalents of TEMPO, a radical trap, was added to the reaction mixture of 4-biphenyl zinc reagent with Togni's reagent 2 in the presence of 30 mol% CuI. The reaction was monitored by 19F NMR spectroscopy and it showed the formation of trifluoromethylated TEMPO in 80% yield and the trifluoromethylated biphenyl in 16% yield, respectively (eqn (1)). This result indicated that the copper-catalyzed trifluoromethylation reaction was only partially inhibited by TEMPO. In the second experiment, we synthesized a zinc reagent 5 to probe if the radical is indeed involved under the standard reaction conditions. Both directed trifluoromethylated product 6a and cyclized trifluoromethylated products 6b were observed after 20 min at room temperature in 36% and 29% yields, respectively (eqn (2)). These results suggest that the copper-catalyzed trifluoromethylation of aryl zinc reagent might proceed via two parallel mechanisms: one involved a radical pathway and the other involved a non-radical pathway.
 | (1) |
 | (2) |
To gain more insight about the mechanism of the reaction, we conducted a stoichiometric reaction of CuI with Togni's reagent 2 (eqn (3)). A clear blue solution was formed within 1.0 min at room temperature upon mixing 19 mg of CuI with 3.0 equivalents of Togni's reagent 2 in 1.0 mL of DMF. The reaction was monitored by 19F NMR spectroscopy and it showed the formation of CF3I (resonated at δ = −10.5 ppm in 19F NMR), CF3H (resonated at δ = −79.5 ppm in 19F NMR), unreacted reagent 2 and an unknown species 7 (resonated at δ = −30.5 ppm in 19F NMR).
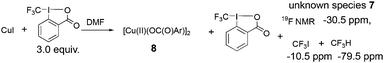 | (3) |
Addition of 10 mL of diethyl ether precipitated a blue solid which was characterized to be bimetallic copper(II) species 8, as determined by X-ray diffraction of its single crystals. This is consistent with previously reported observation by Xi and coworkers.8 After the diethyl ether was removed under vacuum, the DMF solution of the reaction mixture was treated with 1.0 equivalent of 4-biphenyl zinc reagent. After 20 min, the peak at −30.5 ppm disappeared and a new peak at −62.6 ppm which corresponds to the trifluoromethylated biphenyl formed in 47% yield, as determined by 19F NMR spectroscopy (Fig. 1).
We speculated that the unknown species at −30.5 ppm might be [CF3Cu]. To probe if this is the case, we independently prepared [CF3Cu] by a method reported by Grushin.9 Interestingly, stoichiometric reactions of independently prepared [CF3Cu] with 1.0 equivalent of 4-biphenyl zinc reagent 3 in DMF in the presence of oxidants such as air, Ag2CO3, K2S2O8 or AgOTf did not generate the coupled product. When PhI(OAc)2 was used as the oxidant, the trifluoromethylated biphenyl was formed in 20% yield. These experiments suggest that the unknown species at −30.5 ppm might not be [CF3Cu].
Togni reported that reaction of Togni's reagent 2 with Zn(NTf)2 resulted in the formation of [Zn(2)2(NTf)2]+, which showed a shift of the CF3 signal of the hypervalent iodine species from −33.0 ppm to −26.9 ppm in the 19F NMR spectroscopy. We think that a similar complex might be formed when Togni's reagent 2 was reacted with CuI in DMF. To figure out structural information of this transient reactive species at −30.5 ppm, we carefully monitored the diluted reaction mixture of CuI with Togni's reagent 2 in DMF by electrospray ionization tandem Mass spectroscopy (ESI-MS). The typical isotopic distributions of the ion species containing copper helped us the screen the reactive Cu-complexes from the ESI-MS spectra at different time.10 The ESI-MS experimental results revealed that after adding CuI, Togni's reagent 2 to DMF solution, the major peak in the ESI-MS spectrum was the signal of Cu(II) with 2-iodobenzoic acid, which remained inert through the ESI-MS experiments. Interestingly, a new signal with a m/z = 451.6, which was assigned as the signal of [2·Cu(I)·DMF]+, appeared two minutes after addition of CuI, Togni's reagent 2 to DMF solution. This peak slowly increased and finally disappeared after nearly 2.5 h (Fig. 2). These results indicated that this species might be the active transient species that was observed in the 19F NMR spectroscopy, which is consistent with Togni's observation for the formation of [Zn(2)2(NTf)2]+.
 | ||
| Fig. 2 The expanded ESI-MS spectra of: (a) the diluted reaction solution at reaction time 10 min; (b) the diluted reaction solution after 3 hour, in positive ion mode. | ||
Based on these preliminary results, we propose the reaction mechanism for copper-catalyzed trifluoromethylation of organic zinc reagent with Togni's reagent 2, as outlined in Fig. 3. Reaction of CuI with Togni's reagent 2 formed an intermediate 7 and bimetallic copper(II) species 8. Transmetallation of the aryl group from organic zinc reagent to intermediate 7 generated intermediate 9. Directed reductive-elimination from this hypervalent iodine species affords the trifluoromethylated arene (pathway A). Alternatively, intermediate 9 may undergo a homolytic C–I bond scission to form a CF3˙, which will recombine with aryl group to form trifluoromethylated arene (pathway B) or a hemolytic C–I bond scission to form a R˙, which then recombine with the trifluoromethyl group to form the final product (pathway B′). However, the detailed mechanism for the formation of the final product is more complicated and more experiments are required to elucidate this process.
 | ||
| Fig. 3 Proposed mechanism for copper-catalyzed trifluoromethylation of organic zinc reagents with Togni's reagent 2. | ||
In summary, a copper-catalyzed efficient trifluoromethylation of organic zinc reagents is developed. The reaction conditions are mild and various functional groups such as ester, cyano, protected ketone and amide were compatible. Initial mechanistic studies indicated that the aryl group from the zinc reagent is transferred to hypervalent iodine to form a tri-substituted hypervalent iodine intermediate. Consequent reductive-elimination via a concerted bond-forming step and/or trifluoromethyl radical pathway from this intermediate generates the trifluoromethylated arenes. Further investigation to expand the scope of the substrates and clarify the reaction mechanism are currently undergoing in our laboratory.
Acknowledgements
The authors gratefully acknowledge Prof. Dr Qilong-Shen of Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences for his fruitful discussions and the SIOC for financial support.Notes and references
- W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed; J. P. Béguéand D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley-Interscience, New Jersey, 2008 CrossRef; K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305 CrossRef; In Fluorine in Medicinal and Chemical Biology, ed. I. Ojima, Wiley-Blackwell, Chichester, 2009 CrossRef PubMed; I. Ojima, J. Org. Chem., 2013, 78, 6358 CrossRef PubMed.
- J.-P. Bégué and D. Bonnet-Delpon, J. Fluorine Chem., 2006, 127, 992 CrossRef CAS PubMed; K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 237 RSC; R. Filler and R. Saha, Future Med. Chem., 2009, 1, 777 CrossRef PubMed; D. O'Hagan, J. Fluorine Chem., 2010, 131, 1071 CrossRef PubMed; J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef PubMed.
- For recent reviews on trifluoromethylations, see: M. A. McClinton, Tetrahedron, 1992, 48, 6555 CrossRef CAS; J. A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1 CrossRef; A. T. Osleya and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef PubMed; T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef PubMed; X.-F. Wu, H. Neumann and M. Beller, Chem.–Asian J., 2012, 7, 1754 Search PubMed; Z. Jin, G. B. Hammond and B. Xu, Aldrichimica Acta, 2012, 45, 67 Search PubMed; T. Liu and Q. Shen, Eur. J. Org. Chem., 2012, 6679 CrossRef; C.-P. Lv, Q. Shen and D. Liu, Chin. J. Org. Chem., 2012, 32, 1380 CrossRef; F.-L. Qing, Chin. J. Org. Chem., 2012, 32, 815 CrossRef; A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950 CrossRef PubMed; H. Liu, Z. Gu and X. Jiang, Adv. Synth. Catal., 2013, 355, 617 CrossRef; L.-L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef PubMed.
- Selected examples for transition metal-mediated trifluoromethylation, see: Q. Y. Chen and S. W. Wu, J. Chem. Soc., Chem. Commun., 1989, 705 RSC; V. V. Grushin and W. J. Marshall, J. Am. Chem. Soc., 2006, 128, 4632 CrossRef CAS PubMed; V. V. Grushin and W. J. Marshall, J. Am. Chem. Soc., 2006, 128, 12644 CrossRef PubMed; M. Oishi, H. Kondo and H. Amii, Chem. Commun., 2009, 1909 RSC; E. J. Cho, T. D. Senecal, T. Kinzel, Y. Zhang, D. A. Watson and S. L. Buchwald, Science, 2010, 328, 1679 CrossRef PubMed; L.-L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2010, 132, 7262 CrossRef PubMed; X. Wang, L. Truesdale and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 3648 CrossRef PubMed; N. D. Ball, J. W. Kampf and M. S. Sanford, J. Am. Chem. Soc., 2010, 132, 2878 CrossRef PubMed; L.-L. Chu and F.-L. Qing, Org. Lett., 2010, 12, 5060 CrossRef PubMed; N. D. Ball, J. B. Gary, Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 7577 CrossRef PubMed; T. Knauber, F. G. R. Arikan and L. J. Gooßen, Chem.–Eur. J., 2011, 17, 2689 CrossRef PubMed; T. D. Senecal, A. T. Parsons and S. L. Buchwald, J. Org. Chem., 2011, 76, 1174 CrossRef PubMed; T.-F. Liu and Q. Shen, Org. Lett., 2011, 13, 2342 CrossRef PubMed; J. Xu, D.-F. Luo, B. Xiao, Z.-J. Liu, T.-J. Gong, Y. Fu and L. Liu, Chem. Commun., 2011, 47, 4300 RSC; C.-P. Zhang, J. Cai, C.-B. Zhou, X.-P. Wang, X. Zheng, Y.-C. Gu and J.-C. Xiao, Chem. Commun., 2011, 47, 9516 RSC; C.-P. Zhang, Z.-L. Wang, Q.-Y. Chen, C.-T. Zhang, Y.-C. Gu and J.-C. Xiao, Angew. Chem., Int. Ed., 2011, 50, 1896 CrossRef PubMed; O. A. Tomashenko, E. C. Escudero-Adán, M. Martínez Belmonte and V. V. Grushin, Angew. Chem., Int. Ed., 2011, 50, 7655 CrossRef PubMed; H. Morimoto, T. Tsubogo, N. D. Litvinas and J. F. Hartwig, Angew. Chem., Int. Ed., 2011, 50, 3793 CrossRef PubMed; N. D. Litvinas, P. S. Fier and J. F. Hartwig, Angew. Chem., Int. Ed., 2012, 51, 536 CrossRef PubMed; T.-F. Liu, X.-X. Shao, Y. Wu and Q. Shen, Angew. Chem., Int. Ed., 2012, 51, 540 CrossRef PubMed; L.-S. Zhang, K. Chen, G. Chen, B.-J. Li, S. Luo, Q.-Y. Guo, J.-B. Wei and Z.-J. Shi, Org. Lett., 2013, 15, 10 CrossRef PubMed.
- Selected examples for trifluoromethylation via a radical process: Y. Ji, T. Brueckl, R. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond and P. S. Baran, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14411 CrossRef CAS PubMed; D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224 CrossRef PubMed; Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2012, 134, 9034 CrossRef PubMed; Y. Ye, S. A. Künzi and M. S. Sanford, Org. Lett., 2012, 14, 4979 CrossRef PubMed; Y. Li, L. Wu, H. Neumanna and M. Beller, Chem. Commun., 2013, 49, 2628 RSC; J.-B. Tommasino, A. Brondex, M. Mébielle, M. Thomalla, B. R. Langlois and T. Billard, Synlett, 2002, 1697 CrossRef PubMed.
- J. K. Stille, Angew. Chem., Int. Ed., 1986, 25, 508 CrossRef CAS; P. Knochel and R. D. Singer, Chem. Rev., 1993, 93, 2117 CrossRef; E.-I. Negishi, X.-H. Zeng, Z. Tan, M.-X. Qian, Q. Hu and Z.-H. Huang, Palladium- or Nickel-Catalyzed Cross-Coupling with Organometals Containing Zinc, Aluminum, and Zirconium: The Negishi Coupling, in Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijiere and F. Diederich, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2004 Search PubMed.
- P. Eisenberger, S. Gischig and A. Togni, Chem.–Eur. J., 2006, 12, 2579 CrossRef CAS PubMed; P. Eisenberger, I. Kieltsch, N. Armanino and A. Togni, Chem. Commun., 2008, 1575 RSC; K. Niedermann, N. Früh, E. Vinogradova, M. S. Wiehn, A. Moreno and A. Togni, Angew. Chem., Int. Ed., 2011, 50, 1059 CrossRef PubMed; J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef PubMed.
- Selected examples for trifluoromethylation of arenes and their derivatives using Togni's reagent: E. Mejía and A. Togni, ACS Catal., 2012, 2, 521 CrossRef CAS; Y. Ye, N. D. Ball, J. W. Kampf and M. S. Sanford, J. Am. Chem. Soc., 2010, 132, 14682 CrossRef PubMed; M. S. Wiehn, E. V. Vinogradova and A. Togni, J. Fluorine Chem., 2010, 131, 951 CrossRef PubMed; R. Shimizu, H. Egami, T. Nagi, J. Chae, Y. Hamashima and M. Sodeoka, Tetrahedron Lett., 2010, 51, 5947 CrossRef PubMed; A. Miyazaki, R. Shimizu, H. Egami and M. Sodeoka, Heterocycles, 2012, 86, 979 CrossRef PubMed; S. Cai, C. Chen, Z. Sun and C. Xi, Chem. Commun., 2013, 49, 4552 RSC; J. Xie, X. Yuan, A. Abdukader, C. Zhu and J. Ma, Org. Lett., 2014, 16, 1768 CrossRef PubMed; B. M. Schmidt, S. Seki, B. Topolinski, K. Ohkubo, S. Fukuzumi, H. Sakurai and D. Lentz, Angew. Chem., Int. Ed., 2012, 51, 11385 CrossRef PubMed; Y. Huang, X. Fang, X. Lin, H. Li, W. He, K.-W. Huang, Y. Yuan and Z. Weng, Tetrahedron, 2012, 68, 9949 CrossRef PubMed.
- A. Zanardi, M. A. Novikov, E. Martin, J. Benet-Buchholz and V. V. Grushin, J. Am. Chem. Soc., 2011, 133, 20901 CrossRef CAS PubMed.
- H.-Y. Wang, X. Zhang, G.-S. Liu and Y.-L. Guo, J. Am. Soc. Mass Spectrom., 2013, 24, 761 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1047558. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra02279g |
| This journal is © The Royal Society of Chemistry 2015 |