One-pot synthesis of bicyclic sugar oxazolidinone from D-glucosamine†
Someswara Rao Sanapala and
Suvarn S. Kulkarni*
Department of Chemistry, Indian Institute of Technology-Bombay, Powai, Mumbai, 400076, India. E-mail: suvarn@chem.iitb.ac.in; Fax: +91 22 2576 7152; Tel: +91 22 2576 7166
First published on 20th February 2015
Abstract
Herein we report a one-pot and efficient method for the synthesis of a 1,2-cis fused furanoside bicyclic oxazolidinone derivative of D-glucosamine via pyranose to furanose conversion and concomitant cyclization involving the N-Troc group. The D-glucosamine oxazolidinone derivative was efficiently transformed into oxazolidinones of D-xylosamine, D-allosamine and D-ribosamine.
Introduction
The emergence of antibiotic resistance has become a serious problem worldwide.l Over the past two decades, a few organisms such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant coagulase negative Staphylococci (MR-CNS), penicillin-resistant Streptococcus pneumoniae (PRSP) and vancomycin-resistant Enterococcus (VRE) have developed multidrug resistance which causes serious health problems.2–4 One of the most common approaches for tackling bacterial resistance is to continue modifying the existing classes of antibacterial agents to provide new analogs with improved efficacy. In this regard, oxazolidinones have emerged as a promising class of antibiotics against these life threatening antibiotic-resistant superbugs.2a,5,6 Linezolid, the first approved oxazolidinone based drug, shows excellent activity against Gram-positive bacteria, as well as several anaerobes and Mycobacterium tuberculosis.7Oxazolidinones form an important family of biologically active compounds which were extensively used as versatile intermediates for the synthesis of bioactive amino-alcohols,8 as chiral auxiliaries in asymmetric synthesis,9 and as ligands for metal catalysis.10 Moreover, carbohydrate based bicyclic oxazolidinone derivatives have shown to possess anti-angiogenic activity.11 Inhibition of angiogenesis has become a useful approach towards the development and discovery of anti-tumor agents.12 Given their biological importance, new and efficient methods for accessing diverse oxazolidinone scaffolds are in much demand.
During the course of our studies directed towards synthesis of glycosamine containing glycoconjugates, we serendipitously came across the formation of a 1,2-cis-fused bicyclic furanoside oxazolidinone from D-glucosamine, which is quite difficult to prepare otherwise. There are sporadic reports on the formation of such furanoside oxazolidinone derivatives formed mostly as side products, decomposition products or as components of mixtures.13–17 In 1979, Argoudelis and co-workers,13 in the course of the structure determination of the antibiotic streptozocin via degradation studies, reported that the reaction of streptozocin with 2 N NaOH gave a bicyclic furanoside oxazolidinone. Hammer and co-workers14 reported that chlorozotocin decomposes in aqueous buffer solutions (pH 7.4) at 37 °C to give a complex mixture of six intramolecular five-membered ring carbamate (1,3-oxazolidin-2-one) sugars. Vankar and co-workers reported that selective deprotection of the anomeric O-methyl group of the C2-NHBoc bearing D-lyxofuranoside derivative using tetrafluoroborate results in the formation of the corresponding bicyclic oxazolidinone in good yields.15 Likewise, Chen and Fang16 encountered a similar side reaction of NHBoc participation on a D-glucofuranose skeleton during hydrogenolysis leading to an oxazolidinone derivative as a minor side product. Recently, Murakami17 reported that treatment of N-phenoxycarbonyl-D-glucosamine with a catalytic amount of NaHCO3 in methanol at 70 °C gave an inseparable mixture of 2-N,1-O-carbonyl-glucosamines (pyranose and furanose forms) and 2-N,3-O-carbonyl-glucosamine (pyranose form). For the pyranoside counterparts, Nicolaou and co-workers have reported an efficient methodology from glycals through IBX mediated coupling between allylic alcohols and aryl isocyanates.18 To the best of our knowledge, there is no general and efficient synthesis of 1,2-cis fused furanoside oxazolidinones reported till date.
Results and discussion
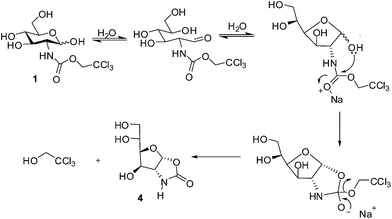
Our synthesis involved preparation of known N-Troc glucosamine derivative 1 (Scheme 1). Following the procedure reported by Schmidt and co-workers, treatment of D-glucosamine with 2,2,2-trichloroethyl chloroformate (TrocCl) in aqueous sodium bicarbonate for 2 h gave 1, which precipitated out as a white solid and characterized as its per-acetate derivative 2.19 Although the reaction worked equally well on a large scale, we faced a practical difficulty of evaporating water from the reaction mixture on a rotary evaporator; the solid froth kept bumping in the flask continuously. To avoid this, methanol was added to make the mixture homogeneous. Though the precipitated product was sparingly soluble in methanol, a clear solution was formed when the reaction mixture was heated at 65 °C on a rotary evaporator under high vacuum. Evaporation of solvents (5 h) followed by per-O-acetylation of the crude product using Ac2O and pyridine afforded a white solid. However, to our surprise, the data of the crystalline product was not identical with 2.The 1H NMR spectrum of the product 3 presented some striking features (see ESI†). (1) The spectrum showed only one set of peaks indicating that it's not a mixture of anomers. (2) The characteristic doublet of doublet (∼4.8 ppm, J = 12 Hz) corresponding to CH2 of N-Troc group was missing. (3) Two types of methyl groups were seen, out of which three signals at δ 2.00, 2.08, and 2.09 ppm were suggestive of the O-acetyl CH3 groups, while a peak at δ 2.57 suggested a different type of acetyl group attached to a more electron withdrawing functionality. (4) The resonance at 6.17 ppm although indicated the presence of an anomeric proton, it's coupling constant (d, J = 5.6 Hz) was not conforming to either 1,2-cis or 1,2-trans pyranoside conformation. (5) The remaining coupling constants of the peaks in the sugar range (4.0–5.8 ppm) were small unlike those of pyranose ring, perhaps indicating a furanose ring. The 1H–1H COSY spectrum showed some interesting features. The correlation between H2 and H3 was absent, and there was no coupling between these two protons (J2,3 = 0) clearly suggesting a dihedral angle of ∼90°. From this data it was clear that the ring was no longer present in the usual 4C1 pyranoside conformation. Intriguingly, the 13C spectrum of the product showed the presence of a carbamate carbonyl at 151.8 ppm in addition to the four O-acetyl carbonyls (170.7, 169.9, 169.8, 168.6 ppm). Again the CH3 methyl signals were found to be grouped in two sets (23.9 and 20.9, 20.8, 20.7). The IR spectrum also showed a strong band at 1799, 1752 and 1715 cm−1 in the carbonyl region. The mass spectrum showed a molecular ion peak at 396.0889 which was again not matching with the N-Troc derivative 2. The spectral data thus indicated a furanoside carbamate derivative devoid of CH2CCl3 of the N-Troc group. The exact structure of the product 3 (ref. 13) was established through its X-ray single-crystal analysis (Fig. 1).†
Since this was a welcome entry to the difficult to obtain 1,2-cis furanoside oxazolidinones, efforts were made to optimize the reaction conditions (Scheme 2). Eventually, we performed this reaction in a one pot manner to obtain the oxazolidinone derivative 4 in 87% yield after column chromatography. Notably, we observed that methanol was not necessary to carry out the transformation. The one-pot reaction also worked well on a multigram scale.
The proposed mechanism of the reaction is depicted below (Scheme 3). In the aqueous basic medium, compound 1 exists as an equilibrating mixture of pyranose and furanose forms through the intermediacy of the open chain form. Due to proximity, the anomeric OH of the furanose attacks the carbonyl of Troc and displaces the trichloroethoxy group via an addition elimination mechanism. The cis-fused bicyclic furanose derivative 4 is preferentially formed due to thermodynamic stability of the product.
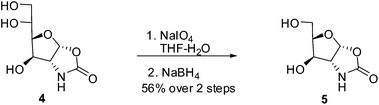
The oxazolidinone derivative 4 could be easily transformed into other sugar derivatives. As shown in Scheme 4, oxidative cleavage of 4 with sodium metaperiodate in THF and water gave the corresponding C-5 aldehyde which upon subsequent reduction with sodium borohydride afforded D-xylosamine derivative 5.
Similarly, compound 4 was transformed into the D-allosamine and D-ribosamine oxazolidinone derivatives as shown in Scheme 5. Treatment of 4 with 2,2-dimethoxy propane and camphorsulfonic acid in CH2Cl2 afforded isopropylidene derivative 6. Triflation of the remaining 3-OH (Tf2O, pyridine) and subsequent SN2 displacement of the formed 3-OTf with sodium benzoate in DMF gave D-allosamine derivative 7. Deprotection of the isopropylidene group by treatment with 80% acetic acid furnished 5,6-diol 8, which upon similar NaIO4 oxidative cleavage followed by borohydride reduction afforded D-ribosamine derivative 9. Removal of the benzoate groups from compounds 8 and 9 by using sodium methoxide in methanol gave compounds 10 and 11 respectively in very good overall yields.
Conclusion
In conclusion, we have successfully synthesized the bicyclic sugar 1,2-oxazolidinone derivatives of D-glucosamine, D-xylosamine, D-allosamine and D-ribosamine in a rapid manner from inexpensive and readily available starting material D-glucosamine. The methodology can be utilized for constructing a small library of oxazolidinones for biological testing.Experimental section
General methods
All reactions were conducted under a dry nitrogen atmosphere. Solvents (CH2Cl2 > 99%, THF 99.5%, acetonitrile 99.8%, DMF 99.5%) were purchased in capped bottles and dried under sodium or CaH2. All other solvents and reagents were used without further purification. All glassware used was oven dried before use. TLC was performed on pre-coated aluminium plates of Silica Gel 60 F254 (0.25 mm, E. Merck). Developed TLC plates were visualized under a short-wave UV lamp and by heating plates those were dipped in ammonium molybdate/cerium(IV) sulfate solution. Silica gel column chromatography was performed using silica gel (100–200 mesh) and employed a solvent polarity correlated with TLC mobility. NMR experiments were conducted on 400 and 500 MHz instrument using CDCl3 (D, 99.8%) or CD3OD (99.8%) or D2O (99.8%) as solvents. Chemical shifts are relative to the deuterated solvent peaks and are in parts per million (ppm). 1H–1H COSY was used to confirm proton assignments. Mass spectra were acquired in the ESI mode using Q-TOF analyser.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ethyl acetate–pet ether) to yield 3 as a crystalline white solid (1.69 g, 93%). [α]20D −43.32 (c 1, EtOH); mp 175–179 °C; IR (CHCl3) ν 3019, 1799, 1752, 1715, 1523, 1374, 1216, 1108, 759 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.17 (d, J = 5.6 Hz, 1H, H-1), 5.67 (d, J = 3.1 Hz, 1H, H-3), 5.23–5.19 (m, 1H, H-5), 4.67 (d, J = 5.6 Hz, 1H, H-2), 4.56 (dd, J = 12.4, 2.4 Hz, 1H, H-6a), 4.33 (dd, J = 9.5, 3.1 Hz, 1H, H-4), 4.09 (dd, J = 12.4, 4.8 Hz, 1H, H-6b), 2.58 (s, 3H), 2.09 (s, 3H), 2.08 (s, 3H), 2.01 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.7, 169.89, 169.87, 168.6, 151.8, 100.1, 76.9, 72.3, 66.8, 64.6, 62.9, 23.9, 20.9, 20.87, 20.76; HRMS-ESI [M + Na]+ calcd for C15H19NNaO10 396.0901, found 396.0909; X-ray data confirmed the furanose ring and the absolute stereochemistry of the compound (see ESI†).
20 ethyl acetate–pet ether) to yield 3 as a crystalline white solid (1.69 g, 93%). [α]20D −43.32 (c 1, EtOH); mp 175–179 °C; IR (CHCl3) ν 3019, 1799, 1752, 1715, 1523, 1374, 1216, 1108, 759 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.17 (d, J = 5.6 Hz, 1H, H-1), 5.67 (d, J = 3.1 Hz, 1H, H-3), 5.23–5.19 (m, 1H, H-5), 4.67 (d, J = 5.6 Hz, 1H, H-2), 4.56 (dd, J = 12.4, 2.4 Hz, 1H, H-6a), 4.33 (dd, J = 9.5, 3.1 Hz, 1H, H-4), 4.09 (dd, J = 12.4, 4.8 Hz, 1H, H-6b), 2.58 (s, 3H), 2.09 (s, 3H), 2.08 (s, 3H), 2.01 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 170.7, 169.89, 169.87, 168.6, 151.8, 100.1, 76.9, 72.3, 66.8, 64.6, 62.9, 23.9, 20.9, 20.87, 20.76; HRMS-ESI [M + Na]+ calcd for C15H19NNaO10 396.0901, found 396.0909; X-ray data confirmed the furanose ring and the absolute stereochemistry of the compound (see ESI†).The crude product was dissolved in methanol (10 mL) and the reaction mixture was cooled to 0 °C. To this sodium borohydride (175.0 mg, 0.81 mmol) in water (3 mL) was added and the reaction mixture was slowly brought to room temperature. The reaction was quenched by addition of acetic acid (0.5 mL) and concentrated on rotor. The residue was purified by column chromatography using 5–10% methanol in ethyl acetate as eluents to give 5 as a hygroscopic white solid (0.4 g, 56%); [α]20D −33.0 (c 0.48, CHCl3); mp 250–255 °C; IR (CHCl3–CH3OH) ν 3439, 2918, 1748, 1654, 1252, 1017, 767 cm−1; 1H NMR (400 MHz, D2O) δ 6.24 (d, J = 5.5 Hz, 1H, H-1), 4.31 (d, J = 5.5 Hz, 1H, H-2), 4.25–4.21 (m, 2H, H-3, H-4), 3.85 (dd, J = 12.0, 3.8 Hz, 1H, H-5a), 3.76 (dd, J = 12.0, 7.5 Hz, 1H, H-5b); 13C NMR (100 MHz, CDCl3) δ 160.4, 104.3, 82.5, 74.4, 64.9, 59.9; HRMS-ESI [M + Na]+ calcd for C6H9NNaO5 198.0373, found 198.0368.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ethyl acetate–pet ether) to obtain 6 as a hygroscopic white foam (2.6 g, 73%); [α]20D −19.01 (c 0.81, CHCl3); IR (CHCl3) ν 3401, 1749, 1652, 1259, 1217, 1115, 1074, 769 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.20 (d, J = 5.4 Hz, 1H, H-1), 5.73 (bs, 1H, NH), 4.36–4.27 (m, 3H, H-2, H-3, H-5), 4.19 (dd, 1H, J = 8.8, 6.2 Hz, 1H, H-6a), 4.10 (dd, J = 7.9, 2.8 Hz, 1H, H-4), 4.00 (dd, J = 8.8, 4.8 Hz, 1H, H-6b), 2.68 (s, 1H, OH), 1.43 (s, 3H, C–CH3), 1.36 (s, 3H, C–CH3); 13C NMR (100 MHz, CDCl3) δ 157.8, 147.6, 110.2, 104.1, 82.2, 75.7, 73.1, 67.8, 64.0, 31.1, 26.7, 25.3. HRMS-ESI [M + H]+ calcd for C10H16NO6 246.0972, found 246.0986.
6 ethyl acetate–pet ether) to obtain 6 as a hygroscopic white foam (2.6 g, 73%); [α]20D −19.01 (c 0.81, CHCl3); IR (CHCl3) ν 3401, 1749, 1652, 1259, 1217, 1115, 1074, 769 cm−1; 1H NMR (400 MHz, CDCl3) δ 6.20 (d, J = 5.4 Hz, 1H, H-1), 5.73 (bs, 1H, NH), 4.36–4.27 (m, 3H, H-2, H-3, H-5), 4.19 (dd, 1H, J = 8.8, 6.2 Hz, 1H, H-6a), 4.10 (dd, J = 7.9, 2.8 Hz, 1H, H-4), 4.00 (dd, J = 8.8, 4.8 Hz, 1H, H-6b), 2.68 (s, 1H, OH), 1.43 (s, 3H, C–CH3), 1.36 (s, 3H, C–CH3); 13C NMR (100 MHz, CDCl3) δ 157.8, 147.6, 110.2, 104.1, 82.2, 75.7, 73.1, 67.8, 64.0, 31.1, 26.7, 25.3. HRMS-ESI [M + H]+ calcd for C10H16NO6 246.0972, found 246.0986.The crude triflate product was dissolved in dimethylformamide (2 mL) and sodium benzoate (0.35 g, 2.45 mmol) was added. The reaction mixture was heated at 100 °C for overnight. The reaction mixture was diluted with water and extracted with CH2Cl2. The organic layers were washed with aq. NaHCO3, brine solution, dried over sodium sulphate and concentrated in vacuo. The residue was purified by column chromatography on silica gel (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ethyl acetate–pet ether) to obtain 7 as hygroscopic yellow solid (130 mg, 62%). [α]20D −87.16 (c 0.43, CHCl3); mp 184 °C; IR (CHCl3) ν 3020, 2923, 2406, 1773, 1725, 1683, 1270, 1216, 770 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.01 (m, 2H, Ar), 7.63–7.61 (m, 1H, Ar), 7.59 (m, 1H, Ar), 6.29 (brs, 1H, NH), 6.18 (d, J = 5.4 Hz, 1H, H-1), 5.35 (d, J = 3.0 Hz, 1H, H-3), 4.44–4.41 (m, 1H, H-5), 4.36–4.32 (m, 2H, H-2, H-4), 4.17 (dd, J = 8.8, 5.9 Hz, 1H, H-6a), 4.10 (dd, J = 8.8, 4.7 Hz, 1H, H-6b), 1.39 (s, 3H, C–CH3), 1.27 (s, 3H, C–CH3); 13C NMR (100 MHz, CDCl3) δ 165.8, 157.6, 134.1, 130.0, 129.9, 129.1, 128.8, 109.9, 103.3, 80.4, 76.9, 76.7, 72.3, 67.4, 62.9, 31.1, 26.9, 25.3; HRMS-ESI [M + K]+ calcd for C17H19NKO7 388.0793, found 388.0785.
6 ethyl acetate–pet ether) to obtain 7 as hygroscopic yellow solid (130 mg, 62%). [α]20D −87.16 (c 0.43, CHCl3); mp 184 °C; IR (CHCl3) ν 3020, 2923, 2406, 1773, 1725, 1683, 1270, 1216, 770 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.01 (m, 2H, Ar), 7.63–7.61 (m, 1H, Ar), 7.59 (m, 1H, Ar), 6.29 (brs, 1H, NH), 6.18 (d, J = 5.4 Hz, 1H, H-1), 5.35 (d, J = 3.0 Hz, 1H, H-3), 4.44–4.41 (m, 1H, H-5), 4.36–4.32 (m, 2H, H-2, H-4), 4.17 (dd, J = 8.8, 5.9 Hz, 1H, H-6a), 4.10 (dd, J = 8.8, 4.7 Hz, 1H, H-6b), 1.39 (s, 3H, C–CH3), 1.27 (s, 3H, C–CH3); 13C NMR (100 MHz, CDCl3) δ 165.8, 157.6, 134.1, 130.0, 129.9, 129.1, 128.8, 109.9, 103.3, 80.4, 76.9, 76.7, 72.3, 67.4, 62.9, 31.1, 26.9, 25.3; HRMS-ESI [M + K]+ calcd for C17H19NKO7 388.0793, found 388.0785.
The crude product was dissolved in methanol (3 mL) and the reaction mixture was cooled to 0 °C using ice bath. To this, sodium borohydride (30.6 mg, 0.81 mmol) in water (1 mL) was added and the reaction mixture was slowly brought to room temperature. The reaction was quenched by addition of acetic acid (0.5 mL) and concentrated on rotor. The residue was purified by column chromatography using 5–10% methanol in ethyl acetate as eluents to give 9 as a hygroscopic white solid (50 mg, 55%); mp 150 °C; IR (CHCl3–CH3OH) ν 3429, 2915, 1750, 1653, 1276, 765 cm−1; [α]20D −35.26 (c 0.43, CHCl3); 1H NMR (500 MHz, CDCl3–MeOD) δ 7.74 (d, J = 7.2 Hz, 2H, Ar), 7.73–7.26 (m, 1H, Ar), 7.16–7.12 (m, 2H, Ar), 5.91 (d, J = 5.2 Hz, 1H, H-1), 4.39 (d, J = 3.1 Hz, 1H, H-3), 4.21–4.17 (m, 2H, H-4, H-5b), 3.95 (d, J = 5.2 Hz, 1H, H-2), 3.87 (m, 1H, H-5a); 13C NMR (125 MHz, CDCl3–MeOD) δ 167.1, 158.8, 133.5, 129.8, 129.5, 128.6, 103.8, 79.6, 74.7, 64.6, 62.8; HRMS-ESI [M + Na]+ calcd for C13H13NNaO6 302.0635, found 302.0635.
Acknowledgements
We thank Department of Science and Technology (Grant no. SR/S1/OC-40/2009), Council of Scientific and Industrial Research (Grant no. 01(2376)/10/EMR-II), Industrial Research and Consultancy Centre IIT Bombay (09IRCC001), and Board of Research in Nuclear Sciences (Grant no. 2013/37C/51/BRNS) for financial support. SRS thanks UGC-New Delhi for a fellowship.References
- (a) R. F. Service, Science, 1995, 270, 724–727 CrossRef CAS; (b) S. B. Levy and B. Marshall, Nat. Med., 2004, 10, S122–S129 CrossRef CAS PubMed.
- (a) C. Walsh, Nature, 2000, 406, 775–781 CrossRef CAS PubMed; (b) C. Walsh, Nat. Rev. Microbiol., 2003, 1, 65–70 CrossRef CAS PubMed.
- (a) C. A. Kauffman, J. Antimicrob. Chemother., 2003, 51, 23–30 CrossRef PubMed; (b) B. Bozdogan and P. C. Appelbaum, Int. J. Antimicrob. Agents, 2004, 23, 113–119 CrossRef CAS PubMed.
- P. C. Appelbaum, Clin. Infect. Dis., 1992, 15, 77–83 CrossRef CAS.
- C. W. Ford, J. C. Hamel, D. Stapert, J. K. Moerman, D. K. Hutchinson, M. R. Barbachyn and G. E. Zurenko, Trends Microbiol., 1997, 5, 196–200 CrossRef CAS.
- (a) S. J. Brickner, M. R. Barbachyn, D. K. Hutchinson and P. R. Manninen, J. Med. Chem., 2008, 51, 1981–1990 CrossRef CAS PubMed; (b) H. Burghardta, K.-L. Schimzb and M. Muller, FEBS Lett., 1998, 425, 40–44 CrossRef; (c) P. Molicotti, S. Ortu, A. Bua, S. Cannas, L. A. Sechi and S. Zanetti, New Microbiol., 2006, 29, 275–280 CAS; (d) M. R. Barbachyn and C. W. Ford, Angew. Chem., Int. Ed., 2003, 42, 2010–2023 CrossRef CAS PubMed.
- (a) J. V. N. Varaprasad, Curr. Opin. Microbiol., 2007, 10, 454–460 CrossRef CAS PubMed; (b) R. C. Moellering, Drug Ther., 2003, 138, 135–142 CAS.
- (a) T. Nagamitsu, T. Sunazuka, H. Tanaka, S. Ōmura, P. A. Sprengeler and A.-B. Smith III, J. Am. Chem. Soc., 1996, 118, 3584–3590 CrossRef CAS; (b) K. Shinozaki, K. Mizuno, H. Oda and Y. Masaki, Bull. Chem. Soc. Jpn., 1996, 69, 1737–1745 CrossRef CAS; (c) S. Knapp, Chem. Soc. Rev., 1999, 28, 61–72 RSC; (d) S. C. Bergmeier and D. M. Stanchina, J. Org. Chem., 1999, 64, 2852–2859 CrossRef CAS PubMed; (e) K. J. Fraunhoffer and M. C. White, J. Am. Chem. Soc., 2007, 129, 7274–7276 CrossRef CAS PubMed.
- (a) R. Noyori, Asymmetric Catalysis in Organic Synthesis, Wiley-Interscience, New York, 1994 Search PubMed; (b) J. Seyden-Pene, Chiral Auxiliaries and Ligands in Asymmetric Synthesis, Wiley-Interscience, New York, 1995 Search PubMed; (c) D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835–876 CrossRef CAS PubMed.
- (a) M. Murakata, H. Tsutsui and O. Hoshino, Org. Lett., 2001, 3, 299–302 CrossRef CAS; (b) H. C. Ma, S. Wu, Q. S. Sun and Z. Q. Lei, Lett. Org. Chem., 2010, 7, 212 CrossRef CAS.
- (a) G. Dorey, P. Léon, S. Sciberras, S. Leonce, N. Guilbaud, A. Pierre, G. Atassi and D. C. Billington, Bioorg. Med. Chem. Lett., 1996, 63, 3045–3050 CrossRef; (b) F. M. Perron-Sierra, A. Pierré, M. Burbridge and N. Guilbaud, Bioorg. Med. Chem. Lett., 2002, 12, 1463–1466 CrossRef CAS.
- (a) J. Folkman, Ann. Surg., 1972, 175, 409–416 CrossRef CAS PubMed; (b) J. Folkman, N. Engl. J. Med., 1989, 320, 1211–1212 CrossRef CAS PubMed.
- P. F. Wiley, R. R. Herr, H. K. Jahnke, C. G. Chidester, S. A. Mizsak, L. B. Spaulding and A. D. Argoudelis, J. Org. Chem., 1979, 44, 9–16 CrossRef CAS.
- C. F. Hammer, R. A. Loranger and P. S. Schein, J. Org. Chem., 1981, 46, 1521–1531 CrossRef CAS.
- A. Kumar, V. R. Doddi and Y. D. Vankar, J. Org. Chem., 2008, 73, 5993–5995 CrossRef CAS PubMed.
- C.-A. Chen and J.-M. Fang, Org. Biomol. Chem., 2013, 11, 7687–7699 CAS.
- T. Murakami, Carbohydr. Res., 2013, 375, 47–54 CrossRef CAS PubMed.
- (a) K. C. Nicolaou, P. S. Baran, Y.-L. Zhong and J. A. Vega, Angew. Chem., Int. Ed., 2000, 39, 2525–2529 CrossRef CAS; (b) J. Zhang, J. Wu, Z. Yin, H. Zeng, K. Khanna, C. Hu and S. Zheng, Org. Biomol. Chem., 2013, 11, 2939–2942 RSC.
- W. Dullenkopf, J. C. Castro-Palomino, L. Manzoni and R. R. Schmidt, Carbohydr. Res., 1996, 296, 135–147 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H, 13C and 2D NMR spectra and crystallographic data. CCDC 1031306. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra02270c |
| This journal is © The Royal Society of Chemistry 2015 |