Biodegradable polyethylene glycol-based ionic liquids for effective inhibition of shale hydration†
Abstract
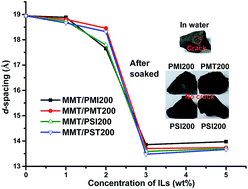
A series of ionic liquids based on polyethylene glycol (PEG) with different molecular weights were prepared for inhibiting shale hydration and swelling. The antiswelling ratio was measured to investigate the effect of different PEG-based ionic liquids on bentonite volume expansion, and it has shown that the ionic liquid based PEG200, i.e. PEG with molecular weight of 200, exhibited superior inhibition. The structures of the PEG200-based ionic liquids were characterized by 1H NMR studies. The XRD results indicated that the PEG200-based ionic liquids intercalated into sodium montmorillonite (Na-MMT) reducing the water uptake by the clay. The formation of complexes of Na-MMT and PEG200-based ionic liquids was also verified by FTIR spectroscopy. Thermal degradation analysis suggested that the PEG200-based ionic liquids accessed the interlamellar spaces of Na-MMT and reduced the water content of the complexes obtained. Moreover, no breaks and collapse were observed on the shale samples after immersion in PEG200-based ionic liquid solutions. All the PEG200-based ionic liquids showed biodegradability and potential application in effective inhibition for clay hydration.

 Please wait while we load your content...
Please wait while we load your content...