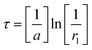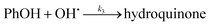DOI:
10.1039/C5RA02226F
(Paper)
RSC Adv., 2015,
5, 36108-36116
Kinetic investigation of photo-catalytic activity of TiO2/metal nanocomposite in phenol photo-degradation using Monte Carlo simulation
Received
4th February 2015
, Accepted 13th April 2015
First published on 14th April 2015
Abstract
Kinetic Monte Carlo simulation was applied to study the kinetics and photo-catalytic activity mechanism of TiO2 anatase, P25, Au/TiO2, Pd/TiO2 and Au–Pd/TiO2 applied in photo-degradation of water pollutants including phenol. The reaction kinetic mechanisms of each aforementioned catalytic system have been obtained. The values of the rate constant for each step of the reaction mechanisms were gained as adjustable parameters by kinetic Monte Carlo simulation. It was shown that the rate constant of formation of electron/hole pairs in metal loaded on titanium dioxide is greater than that of undoped TiO2 because of electron transmission from titanium dioxide to the metal core. The kinetic study of metal performance in M/TiO2 nano composites has demonstrated that the rate constant value of electron transfer from TiO2 to Au is higher than that of Pd and Au–Pd. In this research the kinetic Monte Carlo simulation results agree qualitatively with the existing experimental data for phenol photo-decomposition.
1. Introduction
Titanium dioxide has been widely applied in many fields such as photocatalysis,1,2 water decontamination,3–5 air detoxification,6,7 dye-sensitized solar cells,8,9 and production of hydrogen10,11 due to its high stability in UV light and water. Over the last few years the photo-catalytic activity of TiO2 was successfully enhanced by modification of its surface structure, surface properties and composition.12–17 In numerous investigations the surface modifying has been performed by loading of metal ions and organic polymers.18–30 Metal nanoparticle (NPs) supported on TiO2 has been demonstrated to be a promising method to improve the photo-catalytic performance of titanium dioxide. Contact of TiO2 with metal influences the energetics and the recombination kinetics. On the other hand, the metal/TiO2 composite nanoparticles facilitates charge separation and charge trapping efficiency of TiO2 by rectifying the direction of the holes and electrons flow.31–33 It has been proven the attachment of the metal nanoparticles can shift the Fermi level of TiO2, which results in enhanced photo-catalytic reduction activity.34,35 The application of metal–titanium dioxide nano composite to purification of water and air has also attracted extensive attention during the recent years. For example, TiO2/Pt nanotube has been applied for enhanced photo-catalytic degradation of water pollutant by Huan Chen et al.36 Also Au/TiO2 thin films has demonstrated efficient photo-catalytic removal of water contaminant containing phenol in sunlight.37 At the recent work photo-decomposition of phenol has been promoted using TiO2 doped with Au, Pd, and Au–Pd nanoparticles.38
In this research kinetic parameters and the photo-catalytic mechanism of titanium dioxide and modified titanium dioxide was investigated using computational simulation. Kinetic Monte Carlo (kMC) method has been proven a significant level of application as a powerful tool in modelling of various chemical reactions.39–43 In the present work an efficient method has been employed for identification and comparison of photo-destruction kinetic mechanisms of phenol over TiO2 anatase, TiO2–P25 nano and nano-composite of M/TiO2 (M = Au, Pd and Au–Pd) using kinetic Monte Carlo simulation. The kinetic mechanisms and the rate determining step of abovementioned photo-catalytic systems were obtained. The rate constant of each step was also determined for each systems by kMC method. The kinetic performance of metal in M/TiO2 composite was recognized by theoretical kinetic study. Moreover it was gained the concentration curves versus time for photo-oxidation process of phenol by simulation.
2. Kinetic Monte Carlo method
In order to modelling of the experimental data for photocatalytic removal of phenol by different catalyst, we used the kinetic Monte Carlo simulation developed by Gillespie.44 Kinetic simulations were carried out by the help of the Chemical Kinetic Simulator software, version 1.01.45 In the algorithm of this simulation the reaction mechanism is considered as a series of several reactions:| | |
nN + mM + … → products
| (2.1) |
The input data for the kinetic simulation are the steps, the rate constants of each step (ki) and number of molecules (Ci). The algorithm of this modelling is based on the reaction probability density function (P(τ,i)) which is obtained by Master equation:
| |
P(τ,i) = kiCi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−∑kiCiτ} exp{−∑kiCiτ}
| (2.2) |
The P(τ,i) is two-variable probability density function that can be written as the product of two one-variable probability density functions:
here
Pτ(d
τ) is the probability of happening of the next reaction between times
t +
τ and
t +
τ + d
τ, irrespective of which reaction it could be and
P(
i) shows the probability that the next reaction may be an
Ri reaction that occurs at time
t +
τ.
By the addition theory for probabilities, Pτ(dτ) is obtained by summating of P(τ,i)dτ overall i-values:
| |
 | (2.4) |
That P(i) is gained by substitution of eqn (2.4) in eqn (2.3) as:
| |
 | (2.5) |
These two equations obviously represent the two one-variable density functions in eqn (2.3) that give two-variable density function P(τ,i). By substituting of eqn (2.2) in eqn (2.4) and (2.5) P(τ) and P(i) are afforded as:
| |
P(τ) = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−aτ), 0 ≤ τ < ∞ exp(−aτ), 0 ≤ τ < ∞
| (2.6) |
| |
 | (2.7) |
where we have in summary:
| | |
ai = kiCi, (i = 1,2,…,M)
| (2.8) |
| |
 | (2.9) |
In this special case, P(i) is independent of τ. It is also noted that, both of these one-variable density functions are correctly standardized over their respective explanation:
The idea of this simulation method is creating a random value of τ in accord with P(τ) in eqn (2.6), then generate a random integer i according to P(i) in eqn (2.7). The result of random pair (τ,i) can be divided according to P(τ,i).
A random value τ can be generated by clearly drawing a random number r1, from the uniform distribution in the unit interval and calculating
| |
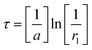 | (2.10) |
Then, a random integer i may be created by drawing another random number r2 from the uniform distribution in the unit interval by taking i to be that integer for which,
| |
 | (2.11) |
In this method, two random numbers r1 and r2 are created and τ and i are calculated by eqn (2.10) and (2.11), respectively.
The simulation was extended by constantly selecting at random among the probability weighted steps in the mechanism and updating the reactants and products populations according to stoichiometry of the selected step, system state variables and reaction rates. The final results were obtained as concentration versus time curves. This stochastic numerical simulation has been used to modelling of several chemical reactions.39–43 In this project kinetic Monte Carlo simulation has been applied to kinetic study of photo-degradation of phenol by various catalytic system containing TiO2 anatase, P25 nano and nanocrystalline of M/TiO2 (M = Au, Pd and Au–Pd).
3. Results and discussion
In this research, it was studied kinetic mechanism of experimental photo-catalytic removal of phenol by a variety of catalytic system containing TiO2,46 P25 nano,38 metal nanoparticles (i.e., Au, Pd, Au–Pd) supported on TiO2 surface.38 Sobczynski and his coworkers have investigated the photo-degradation of phenol using TiO2 anatase.46 Also the photo-decomposition of phenol has been performed using P25 and TiO2 doped with Au, Pd and Au–Pd nanoparticles by Ren Su et al.38 The curves of phenol concentration versus time were obtained in the aforementioned works. In the present study the kinetics simulating of the photo-catalytic activity of TiO2 anatase, P25 nano and M/TiO2 nano composite were carried out using kinetic Monte Carlo method.
3.1. Kinetic simulation of phenol photo-degradation by TiO2 and P25
In order to simulation of phenol photo-oxidation by TiO2 anatase, the input data are temperature (301 K), initial concentration of phenol (5.0 × 10−5 M), initial amount of anatase titanium dioxide (0.05 g),46 the steps of mechanism and rate constants of each step. Several mechanisms have been examined for the photo-degradation of phenol by TiO2 using kinetic Monte Carlo simulation. In the mechanism which has a good fitting with experimental kinetic data, electron/hole pair is formed by irradiation of TiO2. Subsequently the photo-generated hole combines with hydroxyl ions adsorbed on the surface of TiO2 results in formation of hydroxyl radicals. This radical is strong oxidant which can participate in the oxidation of phenol on the TiO2 surface. These steps can be described below:| |
 | (3.1.1) |
| |
 | (3.1.2) |
| |
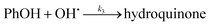 | (3.1.3) |
The snapshots of CKS-Reaction Data Entry windows was presented in Fig. 1. The reaction mechanism and rate constants were put in this windows as shown in Fig. 1. The right rate constants were determine by changing rate determining step. Also the values of rate constants were adjusted until a reasonable fitting of the simulated kinetic data with the experimental data was obtained. Fig. 2 represents a snapshot of the CKS Reaction Conditions window with initial phenol concentrations, the temperature, volume and pressure conditions for the phenol photo-degradation reaction. Suitable fitting of this mechanism with the experimental photo-degradation data was demonstrated in different initial concentrations of phenol ([PhOH]0 = 7.5 × 10−5, 1.0 × 10−4, 1.5 × 10−4, 2.1 × 10−4 M). It is proved that the selected rate constants can be exact by these fittings.
 |
| | Fig. 1 Snapshots of CKS-Reaction Data Entry windows with reaction mechanism, rate constants for the photo-degradation of phenol by titanium dioxide. | |
 |
| | Fig. 2 Snapshot of the CKS Reaction Conditions window for the phenol photo-degradation by titanium dioxide. | |
Also the proposed mechanism has been applied to simulation of phenol photo-decomposition by TiO2–P25 nano (the input data: [PhOH]0 = 4.00 × 10−4 M, [P25]0 = 1 g L−1)38 and the value of the rate constants were obtained as adjustable parameter. The rate constants values for photo-degradation of phenol by TiO2 anatase and P25 nano were listed in Table 1. As seen in the entry 1 of this table, k1, k2 and k3 is constant for five initial concentrations of phenol but there is only the small errors in k2 and k3 for different initial phenol concentration. Furthermore photo-excitation reaction of titanium dioxide (reaction (3.1.1)) is the rate-determining step in phenol photo-destruction by both TiO2 anatase and P25. Therefore, k1 is more important than other rate constants on the rate of phenol photo-degradation. Moreover k1 of P25 nano is increased rather than TiO2 anatase while k2 and k3 are constant for the photo-catalytic reaction using both TiO2 anatase and P25. The difference between k1 of TiO2 anatase and P25 may be described based on higher surface area of P25 nano. Thus, the enhanced total rate of the photo-induced reaction by P25 is attributed to its greater k1 rather than k1 of TiO2 anatase.
Table 1 Rate constants of simulated mechanism for photo-degradation of phenol by titanium dioxide
| Entry |
Catalyst |
k1 (min−1) |
k2 (min−1) |
k3 (min−1) |
| Initial concentration of phenol: 5.0 × 10−5, 7.5 × 10−5, 1.0 × 10−4, 1.5 × 10−4 and 2.1 × 10−4 M. Light source: mercury lamp (180 W).46 Irradiation by UV light source (365 nm LED).38 |
| 1a |
TiO2 anatase |
2.80 × 10−1 |
3.15 ± 0.01 |
3.51 ± 0.1 |
| 2b |
P25 |
1.50 |
3.16 |
3.56 |
Concentrations of phenol versus time curves have been obtained for different initial concentration of phenol in photo-catalytic reaction by anatase titanium dioxide using kinetic Monte Carlo simulation and results were represented in Fig. 3a. As indicated in this figure, simulated data have good agreement with experimental photo-induced data.46 Also there is perfect agreement between kMC simulation and existing experimental data38 as shown in Fig. 3b. These agreements demonstrate that the proposed mechanism can be suitable for study kinetics of photo-degradation of phenol by TiO2.
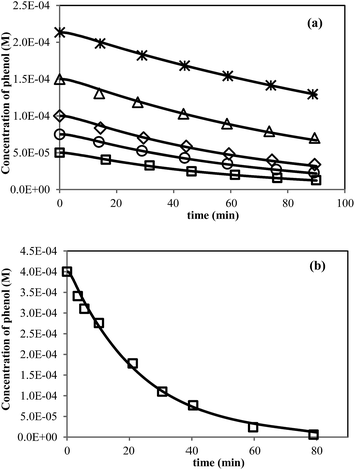 |
| | Fig. 3 Kinetics of phenol removal under photo-excitation of (a) TiO2 anatase (initial concentration of phenol: (□) 5.00 × 10−5, (○) 7.50 × 10−5, (◊) 1.00 × 10−4, (Δ) 1.50 × 10−4 and (*) 2.1 × 10−4 M). (b) P25 (initial concentration of phenol: 4.00 × 10−4 M). Experimental data (open markers) and kMC simulation data (solid line). | |
The mechanism of phenol photo-oxidation by TiO2 has been shown in Scheme 1. Excitation of electrons from the TiO2 valence band to the conduction band is done by irradiation of titanium dioxide band-gap and the holes are create in the valence band. These photo-holes are reduced by hydroxyl radical on the TiO2 surface and hydroxyl radical is formed. Subsequently ˙OH initiates phenol oxidation, producing hydroquinone. At the end of this pathway carbon dioxide and water is created by sequential ˙OH attacks to hydroquinone.47
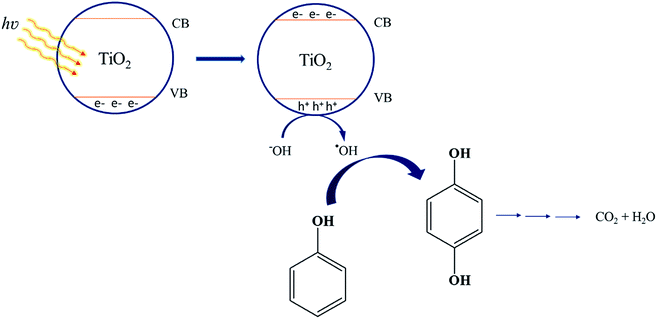 |
| | Scheme 1 The mechanism of phenol photo-degradation over titanium dioxide. | |
3.2. Simulation of photo-catalytic activity of Pd/TiO2, Au/TiO2 and Au–Pd/TiO2 nanocrystallines in phenol photo-destruction
The kinetic Monte Carlo simulation has been performed to finding the mechanism of photo-catalytic removal of phenol by Pd/TiO2 nanoparticles. The input experimental data for the simulation are temperature (298.15 K), initial concentration of phenol (4 × 10−4 M), initial concentration of Pd/TiO2 nanoparticles (1 g L−1),38 the steps of proposed mechanism and rate constants of each step. The values of the rate constants were changed until a perfect fitting of the calculated data with the existing experimental results38 was achieved. Various mechanisms have been examined for the catalytic activity of Pd/TiO2 NPs in phenol photo-oxygenation using kMC simulation. The appropriate mechanism which has been afforded is similar to TiO2 catalytic mechanism as given below:| |
 | (3.2.1) |
| |
 | (3.2.2) |
| |
 | (3.2.3) |
The rate constants k1, k2 and k3 were obtained as adjustable parameters by the simulation and were shown in Table 2 (entry 1).
Table 2 Rate constants of simulated mechanism for photo-decomposition of phenol by M/TiO2 nano composite
| Entrya |
Nano composite |
k1 (min−1) |
k2 (min−1) |
k3 (min−1) |
| Irradiation by UV light source (365 nm LED).38 Size distribution of Pd/TiO2 = 2.9–3.9 nm.38 Size distribution of Au–Pd/TiO2 = 2.9–4.8 nm.38 Size distribution of Au/TiO2 = 3.0–3.7 nm.38 |
| 1 |
Pd/TiO2b |
1.98 ×101 |
3.19 |
4.77 |
| 2 |
Au–Pd/TiO2c |
2.3 × 101 |
3.19 |
4.78 |
| 3 |
Au/TiO2d |
5.72 × 101 |
3.22 |
5.03 |
The proposed mechanism was also applied to simulation of photo-degradation reaction by Pd–Au/TiO2 and Au/TiO2 nano-composites using initial concentration of phenol (4 × 10−4 M) and initial concentration of nano catalyst (1 g L−1).38 The values of the rate constants of the recent simulations are presented in Table 2 (entries 2 and 3). The results of Table 2 indicates the rate-determining step is reaction of photo-induced hole with OH− adsorbed on the surface of M/TiO2 (M = Au, Pd and Au–Pd). The rate constants k2 and k3 are almost equal for the photo-decay reaction of phenol by M/TiO2 nano composites catalyst. Also k1 of the reaction by Pd/TiO2 and Pd–Au/TiO2 are almost constant but enhancement of k1 is demonstrated in the photo-catalytic reaction of Au/TiO2. There are some differences in the kinetic mechanism of phenol photo-decomposition by TiO2 and M/TiO2 composites. For example rate determining step is the first step of mechanism in the reaction with TiO2 and it is the second step in the reaction by M/TiO2 composites. Furthermore k1 of M/TiO2 nano composites are obviously higher than TiO2 and P25. Therefore the rate of phenol photo-degradation is improved using TiO2 loaded with Au, Pd and Au–Pd due to increasing of k1. The increasing in k1 of M/TiO2 nano composites rather than TiO2 can be described based on immediate transmission of the photo-generated electrons from TiO2 to the metal nanoparticle and efficient separation of photo-induced electron/hole pairs. Also enhancing k1 of Au/TiO2 rather than Pd/TiO2 can be attributed to the more appropriate electron transfer level of Au combined with TiO2 than Pd/TiO2.
The curves of phenol concentrations versus time have been obtained for the photo catalytic reaction by Pd/TiO2, Au–Pd/TiO2 and Au/TiO2 using kinetic Monte Carlo simulation. The results were illustrated in Fig. 4. As represented in this figure, there is well agreement between simulation data and experimental photo-induced data.38
 |
| | Fig. 4 Kinetic data of phenol photo-degradation by (a) Pd/TiO2, (b) Au–Pd/TiO2 and (c) Au/TiO2 nano composites. Experimental data (open squares) and kMC simulation data (solid line). | |
3.3. The investigation of Au/TiO2 performance in phenol photo-decomposition
The role of Au/TiO2 in the photo-degradation reaction was investigated by kinetic Monte Carlo simulation using the available experimental data.37 The study was carried out to simulation of 1% gold loading on the TiO2 surface (1% Au/TiO2).37 The input data for the simulation are temperature (298.15 K), initial concentration of phenol (3.19 × 10−4 M),37 the steps of suggested mechanism and rate constants of each step. The values of rate constants were adjusted until a reasonable agreement was observed between the simulated and experimental data.37 Different mechanisms have been studied for the photo-decomposition assay by Monte Carlo simulation. The mechanism which has been afforded by kinetic Monte Carlo simulation can be written as:| |
 | (3.3.1) |
| |
 | (3.3.2) |
| |
 | (3.3.3) |
| |
 | (3.3.4) |
In the first step of the above mechanism, titanium dioxide is activated at the photo-induced condition and electron/hole pairs is created. The rate constant of this step is k1 (reaction (3.3.1)). Afterward the photo-generated electrons transfer to Au nanoparticle by reaction (3.3.2) with the rate constant k2. At the next step the holes move to the nano TiO2 surface, participating in an oxidation reaction (3.3.3) and ˙OH is formed by the rate constant k3. Finally phenol is oxidized by the produced hydroxyl radical (reaction (3.3.4), rate constant = k4). This proposed mechanism was also used to simulation of phenol photo-degradation by 2% Au/TiO2.37
The rate constants were obtained as adjustable parameters using kinetic Monte Carlo simulation. The amounts of the rate constants represents the rate-determining step in the aforementioned mechanism is photo-excitation of TiO2 as shown in Table 3. Furthermore high value of k2 demonstrates the second step is occurred very fast that it proves immediate transmission of the photo-generated electrons from TiO2 to gold nanoparticle. Therefore an efficient separation of photo-induced electron/hole pairs is provided and the phenol photo-degradation reaction is accelerated than undoped TiO2. Also as a result of these simulations k1, k2, k3 and k4 are almost constant for this mechanism using 1% and 2% Au/TiO2.
Table 3 Kinetic parameters of simulated mechanism for phenol photo-destruction over Au/TiO2
| Entrya |
Catalytic system |
k1 (min−1) |
k2 (min−1) |
k3 (min−1) |
k4 (min−1) |
| Irradiation source: sunlight. Size distribution of doped catalytic system: 15–20 nm.37 |
| 1 |
1% Au/TiO2 |
4.17 × 10−1 |
14.33 |
3.17 |
5.45 |
| 2 |
2% Au/TiO2 |
4.27 × 10−1 |
14.35 |
3.16 |
5.42 |
Fig. 5 shows concentration of phenol versus time curves for abovementioned mechanism obtained by kinetic Monte Carlo simulation. As can be seen, simulation data have appropriate fitting with experimental data.37 According to these results, proposed mechanism will be suitable to study kinetics of photo-catalytic activity of metal/TiO2 system.
 |
| | Fig. 5 Kinetic data for photo degradation of phenol by (□) 1% Au/TiO2, (○) 2% Au/TiO2. Experimental data (squares and circles mark) and kMC simulation data (solid line). | |
A probable pathway for the photo-catalytic activity of Au/TiO2 was illustrated in Scheme 2. As shown in this scheme irradiation of TiO2 band-gap excites electrons to the conduction band and creates holes in the valence band. The photo-generated electrons transfer to the gold core and are stored on it. Subsequently the photo-induced holes are scavenged by OH− adsorbed on the TiO2 surface, producing ˙OH. Then hydroxyl radical is substituted on the aromatic ring of phenol and hydroquinone is formed. Finally carbon dioxide and water is created by sequential oxidation of hydroquinone.36,48
 |
| | Scheme 2 The probable mechanism of photo-catalytic activity of Au/TiO2 in phenol photo-decomposition. | |
This mechanism and pathway can be used to explanation of photo-catalytic performance of other metals in M/TiO2 systems. For example palladium is also performed to capture of electron in photo condition of Pd/TiO2 nano-composite. According to the kinetic results which obtained by Monte Carlo simulation, enhanced reaction rate of phenol photo-decomposition by Au/TiO2 rather than Pd/TiO2 demonstrates electron migration from conduction band of titanium dioxide to Au is faster than Pd.
The results are shown in this section, the photo-induced electrons and holes can recombine in the absence of metal. Efficient trapping of photo-generated electrons is occurred by loading of metal nanoparticle on the TiO2 surface. The migration of photo-electron to metal core is fast and it is proven using the kinetic results obtained by kMC simulation. These results show that the rate constant of electron transfer step is high value. This process slow down the recombination of electrons and holes and will increase the charge separation efficiency in M/TiO2 nano composites. Consequently more holes are existing for the photo-oxidation reaction and may effectively improve the catalytic efficiency. Furthermore the differences between the values of k1 in these studied catalytic systems demonstrate that k1 depend on the photon flux because the experiments was performed at different photon fluxes.37,38,46 The variances between the rate constant of phenol degradation (final step) in the studied systems (in the Tables 1–3) can be explained by different surface interaction over various catalytic systems. This value is most in Au/TiO2 nano composite.
4. Conclusions
We used kinetic Monte Carlo simulation as an efficient method to predict and study the kinetics and mechanism of photo-catalytic activity of TiO2 anatase, P25 and metal nanoparticle loaded on TiO2 surface in phenol photo-degradation. The kinetic Monte Carlo simulated results display qualitative agreement with the phenol photo-decomposition experimental data for the each above catalytic system. Therefore these proposed mechanisms can be appropriate for the kinetic study of photo-catalytic removal of phenol. Our results have shown the rate constant value of creation of electron/hole pairs is more in M/TiO2 rather than TiO2 in terms of electron transfer from titanium dioxide to the metal core. The kinetic study of metal performance in M/TiO2 nano composite has been proven the electron migration to Au is faster than Pd and Au–Pd.
Acknowledgements
The author is grateful to University of Kashan for supporting this work by Grant no. (256750/I).
References
- S. N. Frank and A. J. Bard, J. Am. Chem. Soc., 1977, 99, 303 CrossRef CAS.
- S. Malato, J. Caceres, A. Aguera, M. Mezcua, D. Hernando, J. Vial and A. R. Fernandez-Alba, Environ. Sci. Technol., 2001, 35, 4359 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- J. Nowotny, T. Bak, M. K. Nowotny and L. R. Sheppard, J. Phys. Chem. B, 2006, 110, 18492 CrossRef CAS PubMed.
- J. H. Park, O. O. Park and S. Kim, Appl. Phys. Lett., 2006, 89, 163106 CrossRef PubMed.
- C. H. Ao and S. C. Lee, Chem. Eng. Sci., 2005, 60, 103 CrossRef CAS PubMed.
- Y. Q. Cao, T. He, Y. M. Chen and Y. A. Cao, J. Phys. Chem. C, 2005, 114, 3627 CrossRef.
- B. O. Regan and M. Gratzel, Nature, 1991, 353, 737 CrossRef.
- G. K. Mor, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, Nano Lett., 2006, 6, 215 CrossRef CAS PubMed.
- S. C. Moon, H. Mametsuka, S. Tabata and E. Suzuki, Catal. Today, 2000, 58, 125 CrossRef CAS.
- J. S. Jang, W. Li, S. H. Oh and J. S. Lee, Chem. Phys. Lett., 2006, 425, 278 CrossRef CAS PubMed.
- M. Anpo, Y. Ichihasi, M. Takeuchi and H. Yamashita, Stud. Surf. Sci. Catal., 1999, 121, 305 CrossRef CAS.
- M. Anpo, Catal. Surv. Jpn., 1997, 1, 1 CrossRef.
- M. Anpo, Y. Ichihasi, M. Takeuchi and H. Yamashita, Res. Chem. Intermed., 1998, 24, 143 CrossRef CAS PubMed.
- M. Anpo and M. Che, Adv. Catal., 1999, 44, 119 CAS.
- A. Kudo and M. Sekizawa, Chem. Commun., 2000, 1371 RSC.
- K. Sayama and H. Arakawa, J. Chem. Soc., Faraday Trans., 1997, 93, 1647 RSC.
- X. Z. Li and F. B. Li, Environ. Sci. Technol., 2001, 35, 2381 CrossRef CAS.
- V. Subramanian, E. Wolf and P. V. Kamat, J. Phys. Chem. B, 2001, 105, 11439 CrossRef CAS.
- F. Boccuzzi, A. Chiorino, M. Manzoli, D. Andreeva, T. Tabakova, L. Ilieva and V. Idakiev, Catal. Today, 2002, 75, 169 CrossRef CAS.
- M. Date, Y. Ichihashi, T. Yamashita, A. Chiorino, F. Boccuzzi and M. Haruta, Catal. Today, 2002, 75, 51 Search PubMed.
- N. Chandrashekharan and P. V. Kamat, J. Phys. Chem. B, 2000, 104, 10851 CrossRef.
- M. Valden, X. Lai and D. W. Goodman, Science, 1998, 281, 1647 CrossRef CAS.
- D. Andreeva, V. Idakiev, T. Tabakova, L. Ilieva, P. Falaras, A. Bourlinos and A. Travlos, Catal. Today, 2002, 72, 51 CrossRef CAS.
- M. Haruta, Catal. Today, 1997, 36, 153 CrossRef CAS.
- A. Furube, T. Asahi, H. Yamashita and M. Anpo, J. Phys. Chem. B, 1999, 103, 3120 CrossRef CAS.
- A. Furube, T. Asahi, H. Masuhara, H. Yamashita and M. Anpo, Res. Chem. Intermed., 2001, 27, 177 CrossRef CAS PubMed.
- A. Furube, T. Asahi, H. Masuhara, H. Yamashita and M. Anpo, Chem. Phys. Lett., 2001, 336, 424 CrossRef CAS.
- A. Furube, T. Asahi, H. Masuhara, H. Yamashita and M. Anpo, Chem. Lett., 1997, 735 CrossRef CAS.
- M. Takeuchi, M. Matsuoka, H. Yamashita and M. Anpo, J. Synchrotron Radiat., 2001, 8, 643 CrossRef CAS.
- M. Jakob, H. Levanon and P. V. Kamat, Nano Lett., 2003, 3, 353 CrossRef CAS.
- T. Hirakawa and P. V. Kamat, Langmuir, 2004, 20, 5645 CrossRef CAS.
- T. Hirakawa and P. V. Kamat, J. Am. Chem. Soc., 2005, 127, 3928 CrossRef CAS PubMed.
- G. R. Bamwenda, S. Tsubota, T. Nakamura and M. Haruta, J. Photochem. Photobiol., A, 1995, 89, 177 CrossRef CAS.
- K. Mogyorosi, A. Kmetyko, N. Czirbus, G. Vereb, P. Sipos and A. Dombi, React. Kinet. Catal. Lett., 2009, 98, 215 CrossRef CAS.
- H. Chen, S. Chen, X. Quan, H. Yu, H. Zhao and Y. Zhang, J. Phys. Chem. C, 2008, 112, 9285 CAS.
- R. S. Sonawane and M. K. Dongare, J. Mol. Catal. A: Chem., 2006, 243, 68 CrossRef CAS PubMed.
- R. Su, R. Tiruvalam, Q. He, N. Dimitratos, L. Kesavan, C. Hammond, J. A. L. Sanchez, R. Bechstein, C. J. Kiely, G. J. Hutchings and F. Besenbacher, ACS Nano, 2012, 6, 6284 CrossRef CAS PubMed.
- P. Zarzycki, J. Colloid Interface Sci., 2007, 315, 54 CrossRef CAS PubMed.
- L. Liau and C. Y. Lin, Colloids Surf., A, 2011, 388, 70 CrossRef CAS PubMed.
- H. M. Jalali, RSC Adv., 2014, 4, 32928 RSC.
- H. Bashiri, Chem. Phys. Lett., 2013, 575, 101 CrossRef CAS PubMed.
- H. Bashiri, H. Moradmand-Jalali and H. Rasa, Prog. React. Kinet. Mech., 2014, 39, 281 CrossRef CAS.
- D. T. Gillespie, J. Comput. Phys., 1976, 2, 403 CrossRef.
- IBM, CSK Chemical Kinetics Simulator 1.01, IBM Almaden Research Center, IBM Corporation, 1995 Search PubMed.
- A. Sobczynski, L. Duczmal and W. Zmudzinski, J. Mol. Catal. A: Chem., 2004, 213, 225 CrossRef CAS PubMed.
- M. Pimentel, N. Oturan, M. Dezotti and M. A. Oturan, Appl. Catal., B, 2008, 83, 140 CrossRef CAS PubMed.
- P. Amaratunga, M. Lee, J. Kim and D. Lee, Can. J. Chem., 2011, 89, 1001 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp{−∑kiCiτ}
exp{−∑kiCiτ}


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−aτ), 0 ≤ τ < ∞
exp(−aτ), 0 ≤ τ < ∞