One-step synthesis of carboxyl-functionalized rare-earth fluoride nanoparticles for cell imaging and drug delivery†
Abstract
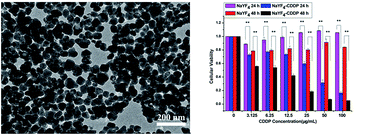
Rare-earth doped UCNPs with a carboxyl coating on the surface have been widely used in many fields of biology, however, the modification of nanoparticles with a carboxyl polymer group is relatively complicated, and thus, fabricating carboxyl polymer-coated UCNPs using a simple method is significant. Herein, we synthesized carboxyl polymer-coated NaYF4:Yb3+/Tm3+ nanoparticles through a hydrothermal route during which methacrylic acid polymerized and bound to the surface of the nanoparticles. Dependence of structure and morphology on the dosage of NaOH was investigated. The polymerization degree of poly(methacrylic acid) and the amount of capping carboxyl group influenced by the dosage of NaOH were also studied. Other carboxyl-functionalized rare-earth fluorides could be obtained by using this method, the mechanism for which was also investigated. Thus, this method was universal for the carboxyl capping of rare-earth doped fluoride nano-materials, and also provided a new approach for carboxylic functionalization of nanoparticles. cis-Dichlorodiammineplatinum(II) (cisplatin, CDDP)-loaded NaYF4:Yb3+/Tm3+ nanoparticles (NaYF4–CDDP) were characterized by transmission electron microscopy, energy-dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy, and CDDP was loaded in the form of Pt–O bonds. Upconversion luminescence images revealed the time course of intracellular CDDP delivery by NaYF4–CDDP. Compared with CDDP alone, the NaYF4–CDDP composite exerted cytotoxic effects on HeLa and MCF-7 cancer cell lines depending more on time and more slowly due to time-dependent cellular uptake and drug release. Non-loaded NaYF4:Yb3+/Tm3+ nanoparticles were also eligible for upconversion luminescence cell imaging. Therefore, the as-prepared NaYF4:Yb3+/Tm3+ nanoparticles allow simultaneous cell imaging and drug delivery as promising anti-cancer theranostic agents.

 Please wait while we load your content...
Please wait while we load your content...