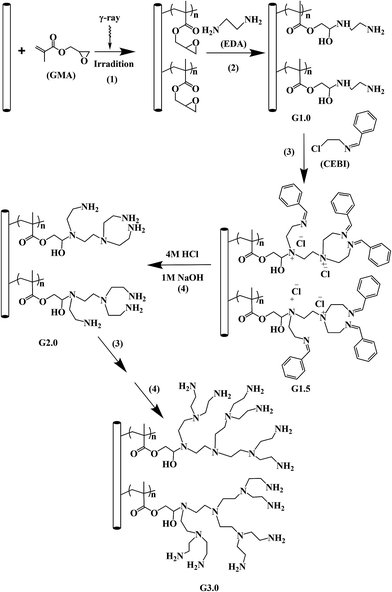
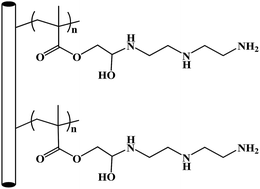
Preparation of polypropylene based hyperbranched absorbent fibers and the study of their adsorption of CO2
Teng Xua,
Qinghua Wua,
Shuixia Chen*ab and
Mengwei Denga
aPCFM Lab, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, PR China. E-mail: cescsx@mail.sysu.edu.cn; Fax: +86-20-84034027
bMaterials Science Institute, Sun Yat-Sen University, Guangzhou 510275, PR China
First published on 25th March 2015
Abstract
Ring-opening copolymerization of aziridine in situ into substrates to introduce polyethyleneimine (PEI) not only required strict experimental conditions but employed the highly toxic monomer aziridine. In this paper, an effective and safe new procedure is developed for hyperbranched structure synthesis by stepwise growth using N-(2-chloroethyl)-benzaldimine as a monomer. Hyperbranched solid amine fibers for CO2 capture were prepared through co-irradiation grafting copolymerization of polypropylene fibers with glycidyl methacrylate, followed by amination with ethylenediamine, Hoffman alkylation with N-(2-chloroethyl)-benzaldimine and then hydrolysis to remove benzaldehyde groups. It was shown that the adsorption performance of the hyperbranched solid amine fibers G2.0 and G3.0 has been greatly improved compared with first generation G1.0 fibers, and the adsorption capacities of G2.0 and G3.0 were 5.35 mmol g−1 and 5.53 mmol g−1 at 30 °C, respectively. The amine utilization of G2.0 fibers could reach 84.1%. These results demonstrate that the branched structure can promote the adsorption capacity and efficiency greatly due to its low mass transfer resistance of CO2, which is more favorable than a linear amination reagent.
1. Introduction
Global warming is mainly attributed to an increase in atmospheric levels of CO2 which is attributed to the unrestrained burning of fossil fuels.1 The most effective method to reduce CO2 emission from the burning of fossil fuels is scrubbing the flue gas stream with an amine solution. But this brings some disadvantages such as corrosion, high regeneration energy and solvent degradation.2,3 To solve these problems, solid amine adsorbents such as amine-treated MCM-41,4,5 SBA-15,6,7 Zeolite 13X8,9 and activated carbon10,11 have been used, attracting much attention. The solid amine adsorbents can retain high adsorption capacities even in the presence of water, compared with microporous or mesoporous adsorbents without amine modification. What is more, they can also be regenerated in mild conditions. The adsorption capacity for CO2 mainly depends on the amine loading and the pore volume of the porous substrates. However, increasing amine loading will block the pore volume of the porous substrate, thus reducing the adsorption capacity of CO2.6,12 So it is of great significance to develop alternative substrates or to improve the amine agents to increase amine loading without affecting adsorption performance.Much work advocating the use of fibrous adsorbents for CO2 adsorption has been published by our group,13–16 demonstrating that fibers have great potential as substrates for CO2 adsorption. Fibrous adsorbents possess the advantages of large external surface area, short transit distance, low pressure drops, and flexibility. Yang15 grafted allylamine onto polyacrylonitrile fibers and obtained an adsorption ability of 6.22 mmol g−1 PAN-AF when the grafting degree was 60.0 wt%. Zhuang16 grafted glycidyl methacrylate onto polypropylene fibers, followed by reaction with triethylenetetramine to make a novel kind of solid amine-containing fibrous adsorbent and obtained an adsorption ability of 4.72 mmol g−1. What is more, the adsorbent could maintain almost constant adsorption behavior within six recycles.
In 1990, Kim put forward the concept of “hyperbranch”, and this then became one of the hottest research topics in Polymer Chemistry.17 Unlike dendritic molecules, hyperbranched molecules do not require uniform structure and high symmetry, thus they are easy to prepare. The most important property is that hyperbranched molecules can have many functional groups that could both improve the hydrophilicity of the material and increase the number of adsorption sites for use as an adsorbent.18,19 Among them, polyethyleneimine (PEI) because of its high amine content and hyperbranched structure was widely reported to modify silicon dioxide20,21 and fibers13,14 that are used for gas separation. The in situ ring-opening polymerization of aziridine into porous silica to introduce PEI for the synthesis hyperbranched polymers has been conventionally carried out and used for CO2 capture. However, on the one hand, the approach has some additional disadvantages such as the use of the highly toxic monomer aziridine and strict experimental conditions.22,23 On the other hand, Li24 has studied the effect of the molecular weight of the PEI on the CO2 capture performance of PEI-nano silica adsorbents and found that a PEI with a high molecular weight leads to unsatisfactory amine utilization because of its high steric hindrance due to the increased viscosity. Thus the development of a PEI with low molecular weight and with an accessible structure will be of benefit to improve its adsorption capacity and the amine efficiency.
In this study, an effective and safe new procedure is developed for hyperbranched structure synthesis by stepwise growth using N-(2-chloroethyl)-benzaldimine as a monomer. The hyperbranched solid amine fibers for CO2 capture were prepared through co-irradiation grafting copolymerization, followed by amination, Hoffman alkylation and hydrolysis. The effects of the branched structure on the adsorption performance of the fiber have been investigated.
2. Experimental
2.1 Materials and reagents
Glycidyl methacrylate (GMA, Sigma-Aldrich CO) was used after the removal of the inhibitor with neutral aluminum oxide. Polypropylene (PP) fibers were provided by Xinshun Special Fiber Company (Zhongshan, China). Methanol, ethanol, triethylamine (TEA), hydrochloric acid (HCl), aluminum chloride hexahydrate (AlCl3·6H2O), magnesium sulfate anhydrous (MgSO4), benzaldehyde, ammonium ferrous sulfate ((NH4)2Fe(SO4)2·6H2O), sodium hydroxide (NaOH), potassium iodide (KI) and ethylenediamine (EDA) were all purchased from Guangzhou Chemical Reagents Factory. Diethylenetriamine (DETA) was purchased from Tianjin Fuchen Chemical Reagents Factory. 2-Chloroethylamine hydrochloride was purchased from Aladdin. Ether was provided by Kaixin Chemical Industry Co. (Hengyang City). All reagents above were analytical grade (AR).2.2 Preparation of hyperbranched solid amine fibers
 | (1) |
 | (2) |
 | (3) |
The preparation scheme of the hyperbranched solid amine fibers is presented in Scheme 1.
In general description, all the modified fibers with amino groups as end groups, G1.0, G2.0 and G3.0, are described as full generation fibers; and those fibers with phenyl groups as the end groups, G0.5, G1.5, and G2.5, are described as half generation fibers.
2.3 Physical and chemical characterization
Infrared (IR) spectra (Tensor-27 spectrometer), Elemental analysis (Elementar, Vario EL), solid state 13C NMR analysis (AVANCE AV, Bruker) and X-ray photoelectron spectroscopy (ESCALAB 250, Thermo-VG Scientific) were used to confirm the structure of the polymers.A thermogravimetric analyzer (Netzsch TG-209) was employed to determine the thermal stability of different full generation samples. The test was carried out from 100 °C to 600 °C using a heating rate of 10 K min−1.
2.4 CO2 adsorption experiment
1.0 g of the fiber sample was placed into a specially designed glass tube (Φ = 13 mm), followed by purging with dry N2 to remove air and excess water. Then a mixture of gases (CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N2 = 1
N2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (volume ratio)) with a flow rate of 30 mL min−1 was introduced. The outlet CO2 concentration was determined by gas chromatography (Techcomp 7900) with a thermal-conductivity detector.
9 (volume ratio)) with a flow rate of 30 mL min−1 was introduced. The outlet CO2 concentration was determined by gas chromatography (Techcomp 7900) with a thermal-conductivity detector.
The adsorption amount was calculated as follows:
 | (4) |
After adsorption, the fiber was regenerated by purging with N2 with a flow rate of 30 mL min−1 at 90 °C.
3. Result and discussion
3.1 Chemical characterization
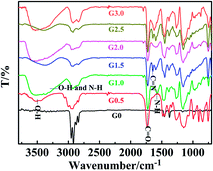
Fig. 1 shows the FT-IR spectrum of grafted fibers at each stage. Compared with G0 fibers, there is a sharp band at 1736 cm−1, which is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of grafted GMA. In addition, a broad and strong absorption band at 3412 cm−1 is attributed to O–H bending vibration. The intensity of this band at 3412 cm−1 increased after amination because it is the overlap of O–H and N–H bending vibrations. The result above proves that EDA was successfully introduced onto the surface of the material. And it is found that there was only a band at 1542 cm−1 for N–H in-plane bending vibration in the range from 1550 cm−1 to 1650 cm−1 for the full generation fiber (G1.0, G2.0 and G3.0), but there was another band at 1640 cm−1 for C
O stretching vibration of grafted GMA. In addition, a broad and strong absorption band at 3412 cm−1 is attributed to O–H bending vibration. The intensity of this band at 3412 cm−1 increased after amination because it is the overlap of O–H and N–H bending vibrations. The result above proves that EDA was successfully introduced onto the surface of the material. And it is found that there was only a band at 1542 cm−1 for N–H in-plane bending vibration in the range from 1550 cm−1 to 1650 cm−1 for the full generation fiber (G1.0, G2.0 and G3.0), but there was another band at 1640 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration for the half generation (G0.5, G1.5 and G2.5), demonstrating the successful preparation of a hyperbranched absorbent.
N stretching vibration for the half generation (G0.5, G1.5 and G2.5), demonstrating the successful preparation of a hyperbranched absorbent.
In order to determine the type of nitrogen and whether the structure was a linear chain or a branched one, elemental analysis, X-ray photoelectron spectroscopy and 13C NMR spectra were carried out and are presented in Tables 1, 2 and Fig. 2, 3, respectively. Compared with G0, both the content of carbon and hydrogen of G0.5 decreased and the calculated grafting degree according to the elemental analysis was 292%, corresponding well to the result measured from weight gain (312%). After amination with EDA, the nitrogen content of G1.0 reached 6.04 mmol g−1, similar to the data (6.67 mmol g−1) calculated by weight gain. Furthermore, it was obvious that the nitrogen content of the full generation increased with increasing generation, proving that the monomer (CEBI) was successfully introduced onto the surface of the fibers. However, the amine number of G2.0 and G3.0 from weight gain was a little lower than that from the elemental analysis, which could be attributed to the difficulty of collection of G2.0 and G3.0 after treatment by strong acid and strong base that led to the brittleness of the fibers. Moreover, with respect to G2.0 and G3.0, incomplete hydrolysis which is confirmed by the 13C NMR spectra where the chemical shifts of the benzyl and imine groups appear at 130 ppm and 140 ppm respectively, gave an explanation for the lower amine content than expected. In addition, as shown in Table 2, combining the elemental analysis data and X-ray photoelectron spectroscopy,26,27 the amount of the different types of amines was calculated. The presence of tertiary amine implicated that the branching can happen to form a hyperbranched polymer.
| Fibers | WGa (g per g raw material) | Element content (wt%) | Amine content (mmol g−1) | ||
|---|---|---|---|---|---|
| C/% | H/% | N/% | |||
a  , where WG represents the weight gain (g per g raw material), Wr and Wp are the weight of the raw material and product, respectively.b The unknown quantities of Cl− would affect the calculation if the raw material refers to G1.5. Thus for the accuracy and convenience, the raw material in this step refers to G1.0.c The raw material in this step refers to G2.0. , where WG represents the weight gain (g per g raw material), Wr and Wp are the weight of the raw material and product, respectively.b The unknown quantities of Cl− would affect the calculation if the raw material refers to G1.5. Thus for the accuracy and convenience, the raw material in this step refers to G1.0.c The raw material in this step refers to G2.0. |
|||||
| G0 | — | 84.30 | 13.66 | 0.00 | — |
| G0.5 | 3.12 | 65.59 | 9.02 | 0.00 | — |
| G1.0 | 0.25 | 50.88 | 9.46 | 8.46 | 6.04 |
| G2.0 | 0.30b | 53.57 | 9.42 | 10.86 | 7.76 |
| G3.0 | 0.22c | 52.74 | 9.34 | 12.09 | 8.64 |
| Fibers | Amine content (mmol g−1) | ||||
|---|---|---|---|---|---|
| Total | Primary | Secondary | Tertiary | Imine | |
| G1.0 | 6.04 | 3.02 | 3.02 | — | — |
| G2.0 | 7.76 | 2.58 | 1.89 | 1.89 | 1.39 |
| G3.0 | 8.64 | 2.84 | 2.29 | 2.02 | 1.48 |
 | ||
| Fig. 3 Typical XPS wide scan spectra for G2.0 and G3.0. The inset shows the characteristic peaks of N 1s at higher resolution. | ||
The results of TG analysis of different generation absorbent fibers are presented in Fig. 4. In the case of PP, there was only one continuous weight loss from 310 °C to 460 °C. In the TG curve of PP-GMA, three distinct weight loss steps were observed at 200 °C, 260 °C, and 350 °C. The first weight loss could be attributed to decomposition of the homopolymer, and the second one between 260 °C and 350 °C was probably related to the degradation of GMA, which also indicated that the monomer GMA was grafted onto the surface of PP. Comparing the G1.0, G2.0 and G3.0 fibers, the thermal stability was G3.0 > G1.0 > G2.0. As is known to all, increasing chain length would reduce the thermal stability. However, G3.0 with the longest chain length showed better stability than the first two generations, overcoming this defect.
3.2 Adsorption property
The cumulative adsorption curves are shown in Fig. 5, and the adsorption capacity and amine utilization efficiency of G1.0, G2.0, G3.0 fibers are shown in Table 3. With the increase of fiber generation, the adsorption capacities increased due to the increase of alkyl amine content, demonstrating the importance of high amine content. | ||
| Fig. 5 The cumulative adsorption curves of G1.0, G2.0, G3.0 fibers (adsorption temperature: 30 °C; N2: 27 mL min−1; CO2: 3 mL min−1). | ||
However, the equilibrium adsorption time increased with the thickening of the adsorption layer which would extend the diffusion time. Moreover, the efficiency of G3.0 decreased to 77.3% while G2.0’s reached 84.1%, which could be ascribed to the longer chain length which increases the steric resistance and disfavors the adsorption. This reminds us of the fact that an appropriate amination reagent which combines alkyl amine content with lower steric resistance should be chosen.
For the purpose of studying the effect of a branched structure on the CO2 adsorption performance, DETA was employed and immobilized onto PP-GMA fiber. Since G2.0 (PP-GMA-EDA-EDA) and G1.0-DETA (PP-GMA-DETA) fibers had similar nitrogen content, the influence of amine content on the adsorption capacity was excluded based on the mechanism in the presence of water vapor.28,29 In addition, G2.0 (PP-GMA-EDA-EDA) had a branched structure, and G1.0-DETA (PP-GMA-DETA) had a linear one. The adsorption capacities and efficiency of G2.0 and G1.0-DETA were compared. As shown in Fig. 6 and Table 3, in the early stages, the adsorption rate of G1.0-DETA was almost the same (up to 20 min) as G2.0’s, and the adsorption capacities of the two materials were 2 mmol g−1 at that stage. The results may be attributed to similar numbers of primary amines on the outer layer. After 20 min, the adsorption rate of G1.0-DETA became slow on account of the higher mass transfer resistance of CO2 into the inner layer for the molecular chain entanglement. Meanwhile, the molecular chain entanglement would also prevent the adsorption sites from reacting with CO2. Both the adsorption capacities and the amine utilization of G2.0 fiber were much higher than G1.0-DETA. The above results demonstrate that the branched structure of G2.0 (PP-GMA-EDA-EDA) is more favorable for adsorption in the presence of water than the linear structure of G1.0-DETA.
 | ||
| Fig. 6 The cumulative adsorption curves of G2.0 and G1.0-DETA fibers (adsorption temperature: 30 °C; N2: 27 mL min−1; CO2: 3 mL min−1). | ||
| Sample | Alkyl amine (mmol g−1) | Adsorption capacity (mmol g−1) | Efficiency (%) |
|---|---|---|---|
| G1.0 | 6.04 | 4.08 | 67.5 |
| G2.0 | 6.36 | 5.35 | 84.1 |
| G3.0 | 7.15 | 5.53 | 77.3 |
| G1.0-DETA | 6.64 | 3.59 | 54.1 |
Compared with other fibrous adsorbents reported (Table 4), G3.0 showed relatively high adsorption efficiency, demonstrating its potential for practical application. More interestingly, Wu14 has prepared a fibrous adsorbent (PP-GMA-PEI) through grafting GMA onto PP fiber, followed by reaction with PEI. Though PP-GMA-PEI has a similar amine content to G3.0, its adsorption capacity was far below than G3.0’s, further confirming the superiority of our route.
| Substrate | Grafted monomer | Amine | Temperature (°C) | pCO2 (atm) | Adsorption capacity (mmol g−1) | Ref. |
|---|---|---|---|---|---|---|
| a Allylamine was directly grafted onto the polyacrylonitrile fiber (PAN).b PEI was coated onto glass fiber by using epichlorohydrin as cross-linking agent. | ||||||
| PP | GMA | Branched | 30 | 0.10 | 5.53 | This work |
| PP | GMA | PEI | 25 | 0.10 | 2.51 | 14 |
| PP | Am | PEI | 25 | 0.10 | 5.91 | 14 |
| PP | GMA | TETA | 30 | 0.15 | 4.72 | 16 |
| PAN | —a | Allylamine | 22 | 0.15 | 6.22 | 15 |
| Glass fiber | —b | PEI | 30 | 0.24 | 4.12 | 13 |
In order to evaluate the regeneration performance of G3.0 fibers, 8 cycles of adsorption–desorption were carried out, and the results are shown in Fig. 7. After 8 cycles, no significant decrease in CO2 adsorption capacity was observed: the adsorption capacity of adsorbent was maintained above 99%. Furthermore, FT-IR spectra of fresh and regenerated G3.0 fibers are presented in Fig. 8. It is obvious that the regenerated fibers exhibit nearly the same spectra as the fresh. All these results confirmed that G3.0 fibers have great regeneration performance that is of great significance for practical application.
4. Conclusion
Polypropylene based hyperbranched absorbent fibers for CO2 capture were prepared by co-irradiation grafting copolymerization of GMA onto PP fiber, followed by amination, Hoffman alkylation and hydrolysis. The present work has shown that the adsorption performance of G2.0 and G3.0 is greatly been improved compared with G1.0, and the adsorption capacities of G2.0 and G3.0 were 5.35 mmol g−1 and 5.53 mmol g−1 at 30 °C, respectively. In particular, a branched structure can promote the adsorption capacity and efficiency greatly due to its low mass transfer resistance of CO2, which is more favorable than linear amination reagent. Furthermore, hyperbranched absorbent fibers could be easily and completely regenerated under mild conditions and are stable in cyclic operations for 8 cycles.Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Grant no. 51173211, 51473187).References
- D. Aaron and C. Tsouris, Sep. Sci. Technol., 2005, 40, 321–348 CrossRef CAS.
- L. Espinal, D. L. Poster, W. Wong-Ng, A. J. Allen and M. L. Green, Environ. Sci. Technol., 2013, 47, 11960–11975 CrossRef CAS PubMed.
- L. Dumée, C. Scholes, G. Stevens and S. Kentish, Int. J. Greenhouse Gas Control, 2012, 10, 443–455 CrossRef PubMed.
- U. Patil, A. Fihri, A. H. Emwas and V. Polshettiwar, Chem. Sci., 2012, 3, 2224–2229 RSC.
- Y. Belmabkhout, R. Serna-Guerrero and A. Sayari, Ind. Eng. Chem. Res., 2010, 49, 359–365 CrossRef CAS.
- A. Zhao, A. Samanta, P. Sarkar and R. Gupta, Ind. Eng. Chem. Res., 2013, 52, 6480–6491 CrossRef CAS.
- S. Hao, H. Chang, Q. Xiao, Y. Zhong and W. Zhu, J. Phys. Chem. C, 2011, 115, 12873–12882 CAS.
- F. Su, C. Lu, S. C. Kuo and W. Zeng, Energy Fuels, 2010, 24, 1441–1448 CrossRef CAS.
- D. P. Bezerra, R. S. Oliveira, R. S. Vieira, C. L. Cavalcante Jr and D. C. S. Azevedo, Adsorption, 2011, 17, 235–246 CrossRef CAS.
- M. Keramati and A. A. Ghoreyshi, Phys. E, 2014, 57, 161–168 CrossRef CAS PubMed.
- C. Zhang, W. Song, G. Sun, L. Xie, J. Wang, K. Li, C. Sun, H. Liu, C. E. Snape and T. Drage, Energy Fuels, 2013, 27, 4818–4823 CrossRef CAS.
- W. J. Son, J. S. Choi and W. S. Ahn, Microporous Mesoporous Mater., 2008, 113, 31–40 CrossRef CAS PubMed.
- P. Li, B. Ge, S. Zhang, S. Chen, Q. Zhang and Y. Zhao, Langmuir, 2008, 24, 6567–6574 CrossRef CAS PubMed.
- Q. Wu, S. Chen and H. Liu, RSC Adv., 2014, 4, 27176–27183 RSC.
- Y. Yang, H. Li, S. Chen, Y. Zhao and Q. Li, Langmuir, 2010, 26, 13897–13902 CrossRef CAS PubMed.
- L. Zhuang, S. Chen, R. Lin and X. Xu, J. Mater. Res., 2013, 28, 2881–2889 CrossRef CAS.
- Y. H. Kim, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 1685–1698 CrossRef CAS.
- A. Sunder, M. Krämer, R. Hanselmann, R. Mülhaupt and H. Frey, Angew. Chem., Int. Ed., 1999, 38, 3552–3555 CrossRef CAS.
- J. Wang, C. Q. Li, J. Li and J. Z. Yang, Sep. Sci. Technol., 2007, 42, 2111–2120 CrossRef CAS.
- M. Badaničová and V. Zeleňák, Monatsh. Chem., 2010, 141, 677–684 CrossRef.
- H. Kassab, M. Maksoud, S. Aguado, M. Pera-Titus, B. Albela and L. Bonneviot, RSC Adv., 2012, 2, 2508–2516 RSC.
- P. Lopez-Aranguren, L. F. Vega and C. Domingo, Chem. Commun., 2013, 49, 11776–11778 RSC.
- J. H. Drese, S. Choi, R. P. Lively, W. J. Koros, D. J. Fauth, M. L. Gray and C. W. Jones, Adv. Funct. Mater., 2009, 19, 3821–3832 CrossRef CAS.
- K. Li, J. Jiang, F. Yan, S. Tian and X. Chen, Appl. Energy, 2014, 136, 750–755 CrossRef CAS PubMed.
- Z. Yao, H. Zhang, T. Dong and D. Yan, Journal of Fudan University, 1989, 28, 34–38 CAS.
- W. Zhang, S. Wang, J. Ji, Y. Li, G. Zhang, F. Zhang and X. Fan, Nanoscale, 2013, 5, 6030–6033 RSC.
- Y. Le, D. Guo, B. Cheng and J. Yu, Appl. Surf. Sci., 2013, 274, 110–116 CrossRef CAS PubMed.
- D. M. D’Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |