Bioactive 18(4 → 3)-abeo-abietanoid derivatives from the leaves of Tripterygium wilfordii†
Chao Wangab,
Chuang-Jun Lia,
Jie Maa,
Jing-Zhi Yanga,
Xiao-Guang Chena,
Li Lia and
Dong-Ming Zhang*a
aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China. E-mail: zhangdm@imm.ac.cn; Fax: +86-10-63165227; Tel: +86-10-63165227
bCollege of Pharmacy, Dalian Medical University, Dalian 116044, China
First published on 23rd March 2015
Abstract
Tripterlides A–F (1–6), six new abietane diterpenoid derivatives, together with six known abietane diterpenoids (7–12) were obtained from the leaves of Tripterygium wilfordii. Tripterlides A–C (1–3) were novel 14(13 → 12),18(4 → 3)-diabeo-abietanoids possessing a 6/6/5 tricyclic ring system. These unusual structures, including their absolute configurations, were determined from UV, IR, HRESIMS, 1D and 2D-NMR data and through comparisons of their experimental and calculated electronic circular dichroism (ECD) spectra. A plausible biosynthetic pathway for 1–3 was proposed. Furthermore, in an in vitro bioassay, 5, 6, 8, and 10–12 showed significant cytotoxic effects against five human cell lines, and 6, 8, 10–12 also exhibited moderate inhibitory activities on hypoxia-inducible factor 1 (HIF-1), which is relevant to tumor development.
Introduction
Tripterygium wilfordii Hook. f. is widely distributed in the southern parts of China, and is known as Lei Gong Teng (Thunder God Vine) in traditional Chinese medicine. The roots of T. wilfordii have been traditionally used to treat rheumatoid arthritis, cancer and kill garden insects.1 Recently, an extract, derived from a water–chloroform extract of the roots (the so-called “total multi-glycoside”) has been used in the clinical treatments of rheumatoid arthritis and other inflammatory and autoimmune diseases.2,3 Previous chemical investigations of this plant have revealed the presences of sesquiterpene polyol esters, sesquiterpene alkoids, diterpenes, triterpenes, and lignans.4–7 Triptolide (10), the typical compound of the 18(4 → 3)-abeo-abietane diterpenoids was isolated from the roots of T. wilfordii by Kupchan.8 Since then, several triptolide analogues have been obtained from T. wilfordii.9–12 Anti-inflammatory, immunosuppressive and anti-cancer activities of those diterpene lactone epoxide compounds have been extensively reported.13 These findings prompted us to systematically investigate the leaves of T. wilfordii. As a result, six new abietane derivative tripterlides A–F (1–6), together with six known 18(4 → 3)-abeo-abietanoids were isolated from the ethanolic extract of the leaves of T. wilfordii by several chromatographic technologies (Fig. 1). Their structures were determined by spectroscopic analyses. Tripterlides A–C (1–3) possessed an unusual 6/6/5 tricyclic ring system and α,β-unsaturated-γ-lactone, which might be derived biosynthetically from the 18(4 → 3)-abeo-abietane diterpenoid lactone by migration of the C-14/C-13 bond to the C-14/C-12 as the key step. It is the first time to obtain these abietane derivatives from natural products. The inhibitory effects on hypoxia-inducible factor 1 (HIF-1) in U251-HRE and cytotoxicities against five human cancer cell lines of 1–12 were also evaluated. We present herein the isolation and structural characterization of tripterlides A–F, as well as their bioactivities.Results and discussion
Tripterlide A (1) was obtained as a pale yellow powder. Its molecular formula C20H24O4 was determined by the HRESIMS at m/z 351.1567 [M + Na]+ (calcd for C20H24NaO4, 351.1573), implying 9 degrees of unsaturation. The UV spectrum of 1 exhibited an absorption maximum at 246 nm, which was characteristic of an α,β-unsaturated ketone. The carbonyl (1749, 1701 cm−1) and methyl (1462, 1378 cm−1) groups were also observed in the IR spectrum of 1.The 1H NMR spectrum revealed the presences of 24 protons (Table 1) and indicated the presence of an isopropyl moiety [δH 1.20 (3H, d, J = 7.2 Hz), 1.14 (3H, d, J = 6.6 Hz), 3.04 (1H, m)], a tertiary methyl [δH 0.93 (3H, s)]. Additionally, the oxymethylene [δH 4.84 (2H, m)] of an α,β-unsaturated-γ-lactone was also observed by the analysis of the 1H NMR spectroscopic data. The 13C NMR spectrum of 1 exhibited 20 carbon signals, which could be attributed to three methyls, six methylenes (including one oxygenated), three methines, and eight quaternary carbons (including four sp2 carbons and three carbonyls) by the DEPT spectrum. Analyses of these carbon signals indicated the presences of an α,β-unsaturated-γ-lactone [δC 173.9, 124.7, 163.1, 71.0], an α,β-unsaturated ketone [δC 206.0, 140.2, 176.0], and an isolated ketone [δC 214.2]. These spectroscopic data suggested that compound 1 was a diterpenoid with a tricyclic ring system, similar to the known 18(4 → 3)-abeo-abietane, such as triptolide analogues.8–12
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δHa | δCb | δHa | δCb | δHc | δCd | |
| a In acetone-d6 (600 MHz for 1, 400 MHz for 2).b In acetone-d6 (150 MHz).c In chloroform-d1 (500 MHz).d In chloroform-d1 (125 MHz). | ||||||
| 1 | 2.12 m | 32.1 | 1.82 m | 32.1 | 1.70 m | 31.6 |
| 1.82 m | 1.54 m | 1.59 m | ||||
| 2 | 2.36 m | 18.4 | 2.30 m | 18.5 | 2.41 m | 17.8 |
| 2.26 m | 2.21 m | 2.28 m | ||||
| 3 | — | 124.7 | — | 124.7 | — | 124.9 |
| 4 | — | 163.1 | — | 163.2 | — | 161.5 |
| 5 | 2.64 brd (12.6) | 43.3 | 2.79 brd (13.6) | 42.4 | 2.86 brd (13.0) | 41.4 |
| 6 | 1.99 m | 19.1 | 2.00 m | 19.5 | 1.93 m | 19.1 |
| 1.82 m | 1.76 m | 1.74 m | ||||
| 7 | 2.38 m | 21.0 | 2.20–2.33 m | 20.8 | 2.44 m | 20.1 |
| 2.22 m | ||||||
| 8 | — | 140.2 | — | 139.7 | — | 140.1 |
| 9 | — | 176.0 | — | 176.7 | — | 175.7 |
| 10 | — | 38.1 | — | 38.4 | — | 37.8 |
| 11 | 2.60 dd (18.0, 7.2) | 41.2 | 2.66 dd (18.0, 7.6) | 41.0 | 2.73 dd (18.0, 7.5) | 40.8 |
| 2.12 dd (18.0, 1.8) | 2.08 m | 2.21 dd (18.0, 2.0) | ||||
| 12 | 4.41 m | 49.7 | 4.44 m | 49.3 | 4.58 m | 43.8 |
| 13 | — | 206.0 | — | 206.0 | — | 205.7 |
| 14 | — | 214.2 | — | 214.2 | — | 213.8 |
| 15 | 3.04 m | 42.5 | 3.00 m | 42.3 | — | 77.5 |
| 16 | 1.20 d (7.2) | 18.6 | 1.17 d (7.2) | 18.7 | 1.51 s | 28.1 |
| 17 | 1.14 d (6.6) | 18.2 | 1.13 d (6.8) | 18.3 | 1.47 s | 27.4 |
| 18 | — | 173.9 | — | 173.9 | — | 173.6 |
| 19 | 4.84 m | 71.0 | 4.84 m | 70.9 | 4.70 m | 70.3 |
| 20 | 0.93 s | 19.2 | 1.07 s | 19.5 | 1.02 s | 19.5 |
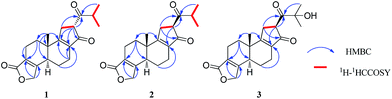
The HMBC correlations of H-1/C-3 (δC 124.7); H-2/C-3 (δC 124.7), and C-4 (δC 163.1); H-19/C-3 (δC 124.7), and C-4 (δC 163.1); and H-20/C-1 (δC 32.2), C-5 (δC 43.4), and C-10 (δC 38.1) established ring A, and confirmed the locations of the α,β-unsaturated-γ-lactone and the tertiary methyl [H-20 (δH 0.93)], respectively (Fig. 2). The ring B also could be established by the long-range correlations between H-6 and C-8 (δC 140.2); H-7 and C-8 (δC 140.2), C-9 (δC 176.0); H-20 and C-9 (δC 176.0) observed in the HMBC spectrum. The 1H–1H COSY correlation of H-11/H-12, and the HMBC correlations of H-11/C-9 (δC 176.0), and C-13 (δC 206.0); and H-12/C-8 (δC 140.2), C-9 (δC 176.0), and C-13 (δC 206.0) established the unusual five membered ring C (Fig. 2). The 1H–1H COSY correlations of H-15/H3-16, and H3-17 confirmed the isopropyl moiety, and the HMBC correlations of H-15/C-14 (δC 214.2), and H-16/C-14 (δC 214.2) established the isobutyl ketone group. The HMBC long-range correlations of H-11/C-14, and H-12/C-14 suggested that the isobutyl ketone was connected to C-12 of ring C.
It was reported that plenty of abietane type diterpenoids including ent-abietanes and abietanes were isolated from various plants.14–19 In consideration of the chemical investigations of T. wilfordii, several abietanes and 18(4 → 3)-abeo-abietanes were obtained.8–12 The 5α-H, 20β-CH3 and α,β-unsaturated-γ-lactone were the structural characteristics of these 18(4 → 3)-abeo-abietanes. The spectroscopic data analyses indicated that compound 1 was derivative of 18(4 → 3)-abeo-abietanes similar to these isolated diterpenoids from T. wilfordii. The NOESY correlations of H-5/H-1a and H-20/H-1b revealed the trans-fused relationship of A/B, same as triptolide analogues.8–12 Therefore, the planar structure of 1 was established as a 14(13 → 12),18(4 → 3)-diabeo-abietane diterpenoid possessing a 6/6/5 tricyclic ring skeleton that likely derived from 18(4 → 3)-abeo-abietane on the basis of above spectroscopic data.
Compound 2 had the same molecular formula as 1 deduced by the HRESIMS at m/z 329.1753. The planar structure of 2 was established same as that of 1 on the basis of the spectroscopic data (Table 1). Therefore, compound 2 was defined to be a stereo-isomer of 1.
Compound 3 showed the molecular formula C20H24O5 by HRESIMS at m/z 345.1701. The 1H NMR spectrum indicated the presences of three methyl groups [δH 1.51, 1.47, 1.02 (each 3H, s)], and the oxymethylene [δH 4.70 (2H, m)] of an α,β-unsaturated-γ-lactone. The 13C NMR spectrum of 3 exhibited 20 carbon signals, which were similar to those of 2, as well as the down shift of C-15 from δC 42.3 (2) to δC 77.5 (3). Analyses of the spectroscopic data (Table 1) of 3 suggested that the planar structure of 3 was also similar to that of 2, except for the hydroxylation of C-15 (δC 77.5).
It was known that triptolide analogues as the natural products containing the 18(4 → 3)-abeo-abietane skeleton were just obtained from T. Wilfordii. Their fine stereo-structures were clarified by X-ray crystallographic analyses. As a result, the stereochemistry of 18(4 → 3)-abeo-abietane skeleton was identified as 5R, 10S.8,20 The absolute configurations of 1–3 were determined as 5R, 10S, 12R (1), 5R, 10S, 12S (2), 5R, 10S, 12R (3) by comparisons of the experimental ECD spectra and calculated ECD data using the time-dependent density functional theory (TD-DFT) method at the B3LYP/6-31G(d) level.
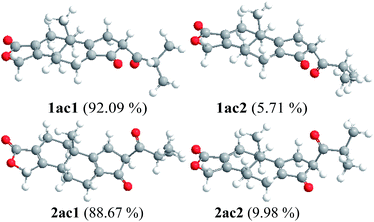
The calculated ECD curves of 5R, 10S, 12R (1a), 5R, 10S, 12S (2a) matched well with experimental data of 1 and 2, respectively (Fig. 3 and 4). Additionally, the conformations analyses of 1a and 2a (Fig. s29 and s30†) suggested that the optimized conformations 1ac1 (92.09%) and 2ac1 (88.67%) were major conformations, which have important roles in the calculated ECD of 1a and 2a. Meantime, it was found that the calculated ECD of conformations 1ac2 (5.71%) and 2ac2 (9.98%) also matched very well with experimental data, which were illustrated in Fig. 3, 4, and 5.
 | ||
| Fig. 3 Calculated ECD spectra of 5R, 20S, 12R (1a), and one of the optimized conformations (1ac2), and the experimental ECD spectrum of 1. | ||
 | ||
| Fig. 4 Calculated ECD spectra of 5R, 20S, 12S (2a), and one of the optimized conformations (2ac2), and the experimental ECD spectra of 2, and 3. | ||
Plausible biogenetic pathways to compounds 1–3 are proposed as shown in Scheme 1. The biosynthetic precursor of 1–3 could be traced back to a proposed 18(4 → 3)-abeo-abietane type (I). After protonation, I would undergo pinacol rearrangement to give the key intermediates II and III, which would readily produce 1–3 respectively by loss of a proton and oxidation of C-13 (Scheme 1).
Compound 4, was obtained as a pale yellow solid. Its molecular formula was established as C19H22O5 by the HRESIMS with the ion peak at m/z 331.1543 [M + H]+ (calcd for 331.1540), implying 9 unsaturation degrees. The UV spectrum exhibited the absorption of unsaturated ketone (λmax 243 nm). 1H NMR (CDCl3, 600 MHz) spectrum (Table 2) indicated the presences of one isopropyl moiety [δH 0.953 (3H, d, J = 6.6 Hz), 0.946 (3H, d, J = 7.2 Hz), 2.08 (1H, m)], one tertiary-methyl [δH 1.14 (3H, s)], one oxygenated methene [δH 4.82, 4.74 (each 1H, s)], one methine [δH 2.71 (1H, brd, J = 13.8 Hz)], and four methylene groups. 13C NMR spectroscopic data (Table 2) exhibited 19 carbon signals, including one α,β-unsaturated-γ-lactone (δC 173.5, 125.8, 162.0, 70.3), and one α,β-unsaturated diketone (δC 156.3, 162.5, 200.8, 200.7). On the basis of above spectroscopic data, compound 4 was established as a nor-diterpenoid with 19 carbons, which was similar to the abietane diterpenoids isolated from T. wilfordii.8–12
| No. | 4 | 5 | 6 | |||
|---|---|---|---|---|---|---|
| δHa | δCb | δHa | δCb | δHa | δCb | |
| a In chloroform-d1 (600 MHz for 4, 5, 400 MHz for 6).b In chloroform-d1 (150 MHz for 4, 5, 100 MHz for 6). | ||||||
| 1 | 2.92 dd | 29.4 | 5.56 d (9.6) | 134.9 | 7.32 d (7.2) | 123.9 |
| (13.2, 6.6) 1.60 m | ||||||
| 2 | 2.47 m | 18.0 | 6.17 d (9.6) | 115.3 | 7.80 d (7.2) | 124.7 |
| 3 | — | 125.8 | — | 123.8 | — | 126.1 |
| 4 | — | 160.0 | — | 157.9 | — | 145.3 |
| 5 | 2.71 brd (13.8) | 42.3 | 3.12 m | 39.9 | — | 127.5 |
| 6 | 2.00 m | 18.4 | 2.27 m | 22.1 | 3.33 d (18.0) | 26.3 |
| 1.78 m | 3.20 d (18.0) | |||||
| 7 | 2.83 dd | 21.1 | 3.37 d (5.4) | 59.4 | 3.62 m | 57.6 |
| (20.4, 6.0) 2.43 m | ||||||
| 8 | — | 156.3 | — | 60.7 | — | 59.6 |
| 9 | — | 162.5 | — | 64.9 | — | 60.9 |
| 10 | — | 35.7 | — | 42.0 | — | 137.4 |
| 11 | — | 200.8 | 3.76 d (3.0) | 59.8 | 3.66 m | 64.0 |
| 12 | — | 78.2 | 3.54 d (3.0) | 54.6 | 3.62 m | 54.9 |
| 13 | — | 200.7 | — | 65.6 | — | 65.9 |
| 14 | 2.08 m | 34.6 | 3.44 d (10.8) | 73.3 | 3.78 d (10.4) | 72.7 |
| 15 | 0.953 d (6.6) | 17.3 | 2.23 m | 28.2 | 2.31 m | 28.5 |
| 16 | 0.946 d (7.2) | 17.3 | 1.02 d (7.2) | 16.9 | 0.95 d (6.0) | 17.0 |
| 17 | — | 173.5 | 0.89 d (7.2) | 17.8 | 1.08 d (6.0) | 17.9 |
| 18 | 4.82 m | 70.3 | — | 170.8 | — | 170.5 |
| 4.74 m | ||||||
| 19 | 1.14 s | 17.98 | 4.82 m | 69.5 | 5.28 d (14.4) | 68.5 |
| 5.21 d (14.4) | ||||||
| 20 | — | — | 1.19 s | 15.3 | — | — |
1H–1H COSY spectrum displayed three separated spin–spin systems (H-1/H-2, H-5/H-6/H-7, H-14/H-15/H-16). In the HMBC spectrum, the long-range correlations of H-1 (δH 2.92)/C-2 (δC 18.0), C-3 (δC 125.8), C-5 (δC 42.3), C-10 (δC 35.7), and C-19 (δC 17.98) established the ring A and confirmed the location of the α,β-unsaturated-γ-lactone. Ring B could be established by the HMBC correlations of H-6/C-5, C-10, H-7/C-8, and C-9. The five membered ring C was deduced by the long-range correlations of H-19/C-9, H-7/C-13, H-14/C-13, and H-16/C-12. The isopropyl moiety was established by the 1H–1H COSY spectroscopic data (H-14/H-15/H-16) and HMBC correlations (H-15/C-14, H-16/C-14). Analyses of the 1D, 2D NMR data revealed that compound 4 was a nor-diterpene with 18(4 → 3)-abeo-abietane skeleton, possessing a 6/6/5 tricyclic ring system.
The absolute configuration of C-12 was determined to be 12S by the comparison of the experimental ECD and calculated CD curves of 4 and its 12-epimer (Fig. 6). Therefore, compound 4 was elucidated as shown, named tripterlide D.
Compound 5, obtained as a white, amorphous solid, had the molecular formula C20H22O6, deduced from the [M + H]+ ion peak at m/z 359.1489. The IR spectrum displayed the absorptions of hydroxyl (3550 cm−1), carbonyl (1764 cm−1), methyl groups (1444, 1371 cm−1). 1H NMR spectrum (Table 2) exhibited the presences of cyclic olefinic bond [δH 5.56 (1H, d, J = 9.6 Hz), 6.17 (1H, d, J = 9.6 Hz)], one isopropyl [δH 1.02 (3H, d, J = 7.2 Hz), 0.89 d (3H, d, J = 7.2 Hz), 2.23 (1H, m)], one tertiary-methyl [δH 1.20 (3H, s)], one oxygenated methene [δH 4.82 (2H, m)], and four oxygenated methines [δH 3.36, 3.44, 3.54, 3.76]. 13C NMR spectroscopic data (Table 2) exhibited 20 carbon signals, which could confirm above moieties and revealed the presence of one α,β-unsaturated lactone [δC 170.8, 157.9, 123.8, 69.5]. Analyses of the spectroscopic data indicated that compound 5 was an 18(4 → 3)-abeo-abietane diterpenoid, which was similar to triptolide.8
The long-range correlations of δH 1.02/δC 65.6 (C-13), δH 1.20/δC 42.0 (C-10), δH 4.82/δC 123.8 (C-3), 157.9 (C-4) observed in the HMBC experiment (Fig. 7) confirmed the 18(4 → 3)-abeo-abietane skeleton. Compared with triptolide, it was obvious that there was one more cyclic olefinic bond in 5. Additionally, the HMBC correlations of δH 5.56/δC 123.8 (C-3), δC 39.9 (C-5), δH 6.17/δC 123.8 (C-3), δC 157.9 (C-4), δC 42.0 (C-10) suggested that the cyclic olifinic bond located at C-1/C-2. The relative configuration could be established by the NOESY experiment. The NOE correlations of H-5(α)/H-7, H-11, H-7/H-14, H-12/H-16 (Fig. 7), and the coupling constant of H-11/H-12 (J = 3.0 Hz) indicated that the relative configuration of 5 was same as triptolide and its analogues.8 Thus, 5 was elucidated as shown, named tripterlide E.
Compound 6 showed the molecular formula C19H18O6 by the HRESIMS. Analyses of the spectroscopic data indicated that 6 possessed the same 18(4 → 3)-abeo-abietane skeleton as that of 5, except for ring A. Compared with 5, one extra olifinic bond (δC 137.4, 127.5) was observed, as well as the absence of CH3-20. Ring A could be established as an aromatic ring by the long-range correlations of δH 7.80/δC 145.3, 137.4, δH 7.32/δC 126.1, 127.5 in its HMBC spectrum. On the basis of its 1D, and 2D NMR spectroscopic data, it was suggested that 6 had the same ring B, and C as 5. Therefore, 6 was established as tripterlide F.
The six known diterpenes were identified as [5aS-(5aα,5bα,8β,9α,9aR*,10aβ)]-4,5a,5b,8,9,10a,11,11a-octahydro-5b,8,9-trihydroxy-5a-methyl-8-(1-methylethyl)-1H-oxireno[8a,9]phenanthro[1,2-c]furan-3(5)-one (7),21 isotriptolide (8),22 triptriolide (9),23 triptolide (10),8 triptolidenol (11),24 tripdiolide (12),8 from their spectroscopic data upon comparisons with values reported in the literatures.
Hypoxia-inducible factor-1 (HIF-1) inhibitory effects of 1–12
Hypoxia-inducible factor-1 (HIF-1), a critical transcription factor to reduce O2 availability, has been demonstrated to be extensively involved in tumor survival, and considered as a potential anticancer target.25,26 In an in vitro bioassay, compounds 6, 8, and 10–12 could inhibit the hypoxia-inducible factor 1 (HIF-1) in U251-HRE with the IC50 9.82, 5.87, 0.02, 0.06, 0.17 μM, respectively (Table 3).| No. | Cytotoxicity IC50 (μM) | HIF-1 inhibitory IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| HCT-116 | HepG2 | BGC-823 | H460 | SK-OV-3 | U251-HRE | |
| 1 | >10 | >10 | >10 | >10 | >10 | >10 |
| 2 | >10 | >10 | >10 | >10 | >10 | >10 |
| 3 | >10 | >10 | >10 | >10 | >10 | >10 |
| 4 | >10 | >10 | >10 | >10 | >10 | >10 |
| 5 | 0.97 | 1.63 | 3.16 | 0.93 | 8.47 | >10 |
| 6 | 0.90 | 0.63 | 0.85 | 0.17 | 2.30 | 9.82 |
| 7 | >10 | >10 | >10 | >10 | >10 | >10 |
| 8 | <0.10 | 0.15 | 0.34 | >10 | >10 | 5.87 |
| 9 | >10 | >10 | >10 | >10 | >10 | >10 |
| 10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | 0.02 |
| 11 | 0.39 | 0.77 | 0.20 | 2.16 | 0.41 | 0.06 |
| 12 | 0.73 | 1.65 | 0.91 | 34.58 | 1.36 | 0.17 |
Cytotoxic activities of compounds 1–12
In bioassay experiments using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method,27 5, 6, 8, and 10–12 exhibited significant cytotoxicities against five human cell lines HCT-116, HepG2, BGC-823, H460, and SK-OV-3 as shown in Table 3.Experimental
General experimental produces
1H-, Optical rotations were measured on a JASCO P-2000 polarimeter. UV spectra were measured on a JASCO V650 spectrophotometer. IR spectra were recorded on a Nicolet 5700 FT-IR microscope instrument (FT-IR microscope transmission). CD spectra were obtained from a JOUAN Mark II spectropolarimeter. NMR spectra were acquired with VNS-600, Bruker-500, and Mercury-400 spectrometers. HRESIMS spectra were collected on an Agilent 1100 series LC/MSD ion trap mass spectrometer. Preparative HPLC was performed on a Shimadzu LC-6AD instrument with a SPD-20A detector, using a YMC-Pack ODS-A column (2 × 25 cm, 5 μm). Column chromatography was performed with silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Qingdao, People's Republic of China) and ODS (50 μm, YMC, Japan). TLC was carried out on glass precoated silica gel GF254 plates. Spots were visualized under UV light or by spraying with 10% sulfuric acid in EtOH followed by heating.Plant material
The leaves of Tripterygium wilfordii were collected in Taining, Fujian, China, in September 2009. A voucher specimen (no. 20090034) was identified by Professor Lin Ma from the Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College and was deposited at the herbarium of the Institute of Material Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, China.Extraction and isolation
Air-dried leaves of Tripterygium wilfordii (50 kg) were extracted with 80% ethanol (400 L × 2 h × 3). After evaporation of EtOH in vacuo, the aqueous residue was diluted with water and then partitioned with EtOAc (30 L × 3). The EtOAc extract (4000 g) was subjected to passage over polyamide by elution with water and 30%, 60%, and 95% EtOH–water in sequence to give fractions A1 (478 g), A2 (743 g), A3 (828 g), and A4 (1000 g). Fraction A1 (478 g) was subjected to column chromatography on silica gel with CHCl3–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–10
0–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 10 fractions (B1–B10). Fraction B7 (52 g) was separated by a silica gel column (200–300 mesh) eluted with CHCl3–MeOH (80
1) to afford 10 fractions (B1–B10). Fraction B7 (52 g) was separated by a silica gel column (200–300 mesh) eluted with CHCl3–MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–10
1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 43 fractions (F1–F43). Subfraction F12 (2.3 g) was passed over an RP-18 column with MeOH–water (20–80%) and finally purified by preparative HPLC (detected at 210 nm, 8 mL min−1) to give 1 (6 mg), 2 (7 mg), 3 (4 mg), 4 (3 mg), 5 (2 mg), 6 (4 mg), 7 (2 mg), 8 (6 mg), 9 (3 mg), 10 (34 mg), 11 (21 mg), and 12 (7 mg).
1) to afford 43 fractions (F1–F43). Subfraction F12 (2.3 g) was passed over an RP-18 column with MeOH–water (20–80%) and finally purified by preparative HPLC (detected at 210 nm, 8 mL min−1) to give 1 (6 mg), 2 (7 mg), 3 (4 mg), 4 (3 mg), 5 (2 mg), 6 (4 mg), 7 (2 mg), 8 (6 mg), 9 (3 mg), 10 (34 mg), 11 (21 mg), and 12 (7 mg).
Structure characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 246 (3.75) nm; IR (microscope) νmax 2958, 2927, 1749, 1701, 1462, 1378, 1020, 753 cm−1; CD (CH3CN) λmax (Δε) 287 (−1.10), 248 (−1.37), 220 (+11.20), 199 (−2.80) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 351.1567 (calcd for C20H24NaO4, 351.1573).
ε) 246 (3.75) nm; IR (microscope) νmax 2958, 2927, 1749, 1701, 1462, 1378, 1020, 753 cm−1; CD (CH3CN) λmax (Δε) 287 (−1.10), 248 (−1.37), 220 (+11.20), 199 (−2.80) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 351.1567 (calcd for C20H24NaO4, 351.1573).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 245 (3.90) nm; IR (microscope) νmax 2968, 2932, 1754, 1702, 1464, 1385, 1071, 1019, 754 cm−1; CD (CH3CN) λmax (Δε) 295 (+1.82), 244 (+9.07), 222 (−11.42), 206 (+3.32) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 329.1753 (calcd for C20H25O4, 329.1747).
ε) 245 (3.90) nm; IR (microscope) νmax 2968, 2932, 1754, 1702, 1464, 1385, 1071, 1019, 754 cm−1; CD (CH3CN) λmax (Δε) 295 (+1.82), 244 (+9.07), 222 (−11.42), 206 (+3.32) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 329.1753 (calcd for C20H25O4, 329.1747).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 245 (3.71) nm; IR (microscope) νmax 3431, 2928, 2852, 1747, 1702, 1460, 1385, 1073, 1021, 756 cm−1; CD (CH3CN) λmax (Δε) 315 (+0.49), 247 (+3.47), 224 (−4.01) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 345.1701 (calcd for C20H25O5, 345.1697).
ε) 245 (3.71) nm; IR (microscope) νmax 3431, 2928, 2852, 1747, 1702, 1460, 1385, 1073, 1021, 756 cm−1; CD (CH3CN) λmax (Δε) 315 (+0.49), 247 (+3.47), 224 (−4.01) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 345.1701 (calcd for C20H25O5, 345.1697).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 243 (4.25) nm; CD (CHCl3) λmax (Δε) 239 (+12.05), 267 (−2.25), 333 (+0.486) nm; 1H NMR and 13C NMR data see Table 2; HRESIMS m/z 331.1543 (calcd for C19H23O5, 331.1540).
ε) 243 (4.25) nm; CD (CHCl3) λmax (Δε) 239 (+12.05), 267 (−2.25), 333 (+0.486) nm; 1H NMR and 13C NMR data see Table 2; HRESIMS m/z 331.1543 (calcd for C19H23O5, 331.1540).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 233 (0.93), 275 (2.12) nm; IR (microscope) νmax 3550, 2966, 2933, 1764, 1444, 1371, 1034, 1020, 745 cm−1; 1H NMR and 13C NMR data see Table 2; HRESIMS m/z 359.1489 (calcd for C20H23O6, 359.1496).
ε) 233 (0.93), 275 (2.12) nm; IR (microscope) νmax 3550, 2966, 2933, 1764, 1444, 1371, 1034, 1020, 745 cm−1; 1H NMR and 13C NMR data see Table 2; HRESIMS m/z 359.1489 (calcd for C20H23O6, 359.1496).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 245 (4.67), 277 (0.24), 286 (0.24) nm; IR (microscope) νmax 3456, 2958, 2919, 1777, 1763, 1467, 1399, 1078, 1038 cm−1; 1H NMR and 13C NMR data see Table 2; HRESIMS m/z 343.1176 (calcd for C19H19O6, 343.1179).
ε) 245 (4.67), 277 (0.24), 286 (0.24) nm; IR (microscope) νmax 3456, 2958, 2919, 1777, 1763, 1467, 1399, 1078, 1038 cm−1; 1H NMR and 13C NMR data see Table 2; HRESIMS m/z 343.1176 (calcd for C19H19O6, 343.1179).Inhibitory effects on hypoxia-inducible factor 1 (HIF-1) in U251-HRE
Compounds 1–12 were tested for their ability to inhibit hypoxia-inducible factor 1 (HIF-1) in U251-HRE cells. This assay was carried out as previously described.28Cytotoxicities assay
Compounds 1–12 were tested for cytotoxicities against HCT-116 (human lung carcinoma), HepG2 (human liver carcinoma), BGC-823 (human stomach carcinoma), H460 (human colon carcinoma), and SK-OV-3 (human ovarian carcinoma) cell lines by means of the MTT method as described in the literature.25Conclusions
In conclusion, twelve abietane derivatives were obtained from the traditional Chinese medicine Tripterygium wilfordii Hook. f. Especially, three novel 14(13 → 12),18(4 → 3)-diabeo-abietane diterpenoids, tripterlides A–C (1–3), were isolated, which possessed 6/6/5 tricyclic ring skeleton that likely derived from 18(4 → 3)-abeo-abietane. It is the first time to report these abietane derivatives isolated from natural products. Additionally, these diterpenoids displayed potential HIF-1 inhibitory effects, which were suggested to be the main bioactive substance of T. wilfordii. On the basis of the structures and bioactivities, preliminary structure & activity relationship was deduced. It was suggested that the epoxy groups of the abietanoids may play important role in the cytotoxicities against cancer cells and HIF-1 inhibitory effects. These findings prompt us to pay more attentions to trace bioactive diterpenoids in chemical studies of medicinal plants.Acknowledgements
This work was supported by the National Key Technology R & D program of the Ministry of Science and Technology of China (no. 2011BAI01B05), the National Natural Science Foundation of China (no. 21132009), and the Beijing Natural Science Foundation (no. 7121010).Notes and references
- Jiangsu New Medical College, Dictionary Traditional Drugs (Zhong Yao Da Ci Dian), Shanghai Scientific and Technical Press, Shanghai, 1977, vol. 2, pp. 2469–2470 Search PubMed.
- M. Xiang and C. L. Zhang, Chin. Tradit. Herbal Drugs, 2005, 36, 458–461 CAS.
- Y. G. Wan, W. Sun, H. L. Zhang, Q. J. Yan, P. Chen, C. H. Dou, H. M. Yang and M. Ge, Nephron Exp. Nephrol., 2010, 114, e7–e14 CrossRef PubMed.
- H. Q. Duan, Y. Takaishi, H. Momota, Y. Ohmoto, T. Taki, M. Tori, S. Takaoka, Y. F. Jia and D. Li, Tetrahedron, 2001, 57, 8413–8424 CrossRef CAS.
- H. Q. Duan, Y. Takaishi, Y. F. Jia and D. Li, Phytochemistry, 2001, 56, 341–346 CrossRef CAS.
- Y. G. Luo, M. Zhou, Q. Ye, Q. Pu and G. L. Zhang, J. Nat. Prod., 2012, 75, 98–102 CrossRef CAS PubMed.
- X. Cao, C. J. Li, J. Z. Yang, B. X. Wei, S. P. Yuan, Y. M. Luo, Q. Hou and D. M. Zhang, Fitoterapia, 2012, 83, 343–347 CrossRef CAS PubMed.
- S. M. Kupchan, W. A. Court, R. G. Dailey, C. J. Gilmore and R. F. Bryan, J. Am. Chem. Soc., 1972, 94, 7194–7195 CrossRef CAS.
- C. P. Zhang, X. Y. Lv, P. C. Ma, Y. Chen, Y. G. Zhang, Z. Yan, G. F. Chen, Q. T. Zheng, C. H. He and D. Q. Yu, Acta Pharmacol. Sin., 1993, 28, 110–115 CAS.
- H. Q. Duan, Y. Takaishi, H. Momota, Y. Ohmoto, T. Taki, Y. F. Jia and D. Li, J. Nat. Prod., 1999, 62, 1522–1525 CrossRef CAS PubMed.
- F. J. Guo, M. L. Xi and Y. C. Li, Tetrahedron Lett., 1999, 40, 947–950 CrossRef CAS.
- S. Lin, X. Y. Yu, H. Q. Que, Z. Chen, D. L. Xie and Y. C. Li, Acta Pharmacol. Sin., 2005, 40, 632–635 CAS.
- J. R. Zheng, K. X. Gu, L. F. Xu, J. W. Gao, Y. H. Yu and M. Y. Tang, Acta Acad. Med. Sin., 1991, 13, 391–397 CAS.
- H. Haba, C. Lavaud, H. Harkat, A. A. Magid, L. Marcourt and M. Benkhaled, Phytochemistry, 2007, 68, 1255–1260 CrossRef CAS PubMed.
- J. M. Amaro-Luis, J. R. Herrera and J. G. Luis, Phytochemistry, 1998, 47, 895–897 CrossRef CAS.
- C. T. Che, T. X. Zhoua, Q. G. Ma, G. W. Qin, I. D. Williams, H. M. Wu and Z. S. Shi, Phytochemistry, 1999, 52, 117–121 CrossRef CAS.
- R. Tanaka, H. Ohtsu and S. Matsunaga, Phytochemistry, 1997, 46, 1051–1057 CrossRef CAS.
- J. Z. Xu, J. Lu, F. Sun, H. Z. Zhu, L. J. Wang, X. Y. Zhang and Z. J. Ma, Phytochemistry, 2011, 72, 1482–1487 CrossRef CAS PubMed.
- N. Crespi-Perellino, L. Garofano, E. Arlandini and V. Pinciroli, J. Nat. Prod., 1996, 59, 773–776 CrossRef CAS.
- Y. Lv, Q. T. Zheng, Z. Y. Tian, P. C. Ma, C. P. Zhang, Y. Chen and J. R. Zheng, Nat. Prod. Res. Dev., 1998, 10, 1–5 Search PubMed.
- J. M. Jung, M. Wickramaratne and M. Hepperle, US Pat. 5972998, 1999.
- D. Q. Yu, D. M. Zhang, H. B. Wang and X. T. Liang, Acta Pharmacol. Sin., 1992, 27, 830–836 CAS.
- P. C. Ma and C. L. Yang, Acta Bot. Sin., 1993, 35, 637–643 CAS.
- F. X. Deng, S. Q. Huang, J. H. Cao, Z. L. Xia, S. Lin, D. Y. Zhu, S. H. Jiang and Y. L. Zhu, Acta Bot. Sin., 1985, 27, 516–519 CAS.
- Z. L. Zhou, Z. G. Luo, B. Yu, Y. Jiang, Y. Chen, J. M. Feng, M. Dai, L. J. Tong, Z. Li, Y. C. Li, J. Ding and Z. H. Miao, Mol. Cancer, 2010, 9, 268 CrossRef PubMed.
- M. Zhang and Z. H. Xu, J. Int. Oncol., 2007, 34, 484–487 Search PubMed.
- L. Yu, J. Z. Yang, X. G. Chen, J. G. Shi and D. M. Zhang, J. Nat. Prod., 2009, 72, 866–870 CrossRef CAS PubMed.
- L. W. Lang, K. Tang, Y. Li, X. Y. Liu, C. Wang and X. G. Chen, China J. Chin. Mater. Med., 2012, 37, 2151–2155 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The spectra including 1D, 2D-NMR, HRESIMS of compounds 1–6 as well as related original ECD calculation data for tripterlides A (1), B (2), and C (3). See DOI: 10.1039/c5ra02174j |
| This journal is © The Royal Society of Chemistry 2015 |