ZnFe2O4 nanoparticles and a clay encapsulated ZnFe2O4 nanocomposite: synthesis strategy, structural characteristics and the adsorption of dye pollutants in water†
Abstract
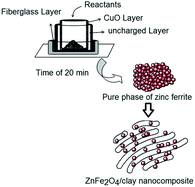
In this work, zinc ferrite nanoparticles were prepared by a one-pot microwave assisted combustion in the solid state with a controllable power up to 900 W and a tunable time of up to 20 min. Then, the ZnFe2O4 nanoparticles were encapsulated in an organoclay using ultrasound assisted impregnation to obtain a zinc ferrite/organoclay nanocomposite as a magnetic heterostructured adsorbent. The combustion reactions were performed in a reaction set-up manufactured from an alumina crucible encircled by a jacket of CuO as a microwave absorbing layer. This workpiece absorbs the distributed microwaves and supplies calcination conditions without any external heat source to prepare a pure phase of zinc ferrite. The effects of the microwave power and the time parameters on the structural and morphological features of the samples are discussed in detail. The adsorption performance of the products for water purification of some dye pollutants was also studied. The UV-Vis results showed that the addition of the as-prepared magnetic nanoparticles to the exfoliated organoclay considerably increased the adsorption of organic dye pollutants from an aqueous solution. Magnetic hysteresis measurements were performed on a vibrating sample magnetometer (VSM) showing the soft paramagnetic properties of the resulting products at room temperature. The structural and morphological analyses were also investigated in detail.

 Please wait while we load your content...
Please wait while we load your content...