Ultrahigh molecular weight, lignosulfonate-based polymers: preparation, self-assembly behaviours and dispersion property in coal–water slurry†
Abstract
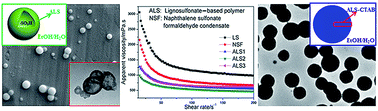
Using a novel and facile method, we synthesized a family of ultrahigh molecular weight, lignosulfonate-based polymers (ALSs) via alkyl chain coupling polymerization. Gel permeation chromatography (GPC) showed a significant increase in weight-average molecular weights (Mws), from 42 800 Da of ALS1 to 251 000 Da of ALS5—one of the highest Mws among reported lignosulfonates (LSs) to date. Functional group content measurements, FTIR and 1H-NMR confirmed the efficient polymerization by nucleophilic substitution coupling mechanism and suggested a straightforward relationship between the polymerization of lignosulfonate (LS) and consumption of phenolic hydroxyl groups. Moreover, hollow nanospheres were obtained via self-assembly of water-soluble ALS and were investigated by DLS, SEM, TEM and AFM. The hollow sphere structure, with a hydrophilic core and a hydrophobic shell, was confirmed by XPS and elemental analysis. Stable, quasi-solid nanospheres were obtained from ALS by the addition of cetyl trimethyl ammonium bromide (CTAB). Furthermore, ALS2, with its relatively high molecular weight, showed unexpectedly better dispersion properties than the raw material LS and naphthalene sulfonate formaldehyde condensate (NSF) for coal–water slurry. The effective polymerization route to improving Mw and the self-assembly from polymer-only ALS provide novel avenues for high-value application of lignin, a sustainable and abundant bioresource.

 Please wait while we load your content...
Please wait while we load your content...