The impact of polymer structure on the adsorption of ionic polyamino acid homopolymers and their diblock copolymers on colloidal chromium(iii) oxide
Abstract
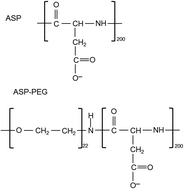
The aim of the presented study was to investigate the influence of the structure and ionic nature of polymers on the adsorption layer architecture. Chromium(III) oxide was used as an adsorbent. The surface behaviour with the addition of different polyamino acids or polyethylene glycol diblock copolymers was analyzed as a function of the solution pH. The analysis of the data obtained from the adsorption and electrokinetic measurements allowed for the proposal of the most probable structures of the polymer adsorption layers found at the solid particle–aqueous polymer solution interface. Moreover, the application of stability measurements enabled the determination of the interactions between the system constituents. Additionally, investigating the architecture of the adsorbed polymer chains on the solid particles is essential for the further applications of the studied macromolecular compounds.

 Please wait while we load your content...
Please wait while we load your content...