Process intensification for tertiary amine catalyzed glycerol carbonate production: translating microwave irradiation to a continuous-flow process†
Daniel O. Nogueira,
Stefânia P. de Souza,
Raquel A. C. Leão,
Leandro S. M. Miranda and
Rodrigo O. M. A. de Souza*
Biocatalysis and Organic Synthesis Group, Chemistry Institute, Universidade Federal do Rio de Janeiro, Bloco A 622, Rio de Janeiro, RJ 21941-909, Brazil
First published on 18th February 2015
Abstract
Different products of interest can be produced from glycerol and glycerol carbonate (GC) has received much attention in recent years because of its physical properties, nontoxicity and water solubility. Herein we report a process intensification protocol for glycerol carbonate production mediated by tertiary amine catalysis where a 24 kg per L per day productivity was obtained with 79% isolated yield and a 5 hour operation time without losing efficiency.
1. Introduction
The biodiesel industry has grown in recent years and has emerged as a leading source of renewable glycerol (∼63%) accounting for 2247 kilo tons in 2013 and an expected market of %2.52 billion by 2020. Personal care, the pharmaceutical industry, food and beverages were the largest application segments for glycerol.1Among the different products of interest that can be produced from glycerol, glycerol carbonate (GC) has received much attention in recent years mainly because of its physical properties (i.e. fp > 240 °C, bp 110–115 °C at 0.1 mm Hg), nontoxicity and water solubility. The applications of glycerol carbonate are wide and can go from solvent to beauty and personal care.
Several strategies can be used to synthesize glycerol carbonate and many of them have already been reported, but just a few can meet the criteria for a industrial manufacturing strategy as pointed out by Ochoa-Gomez and co-workers (Fig. 1).2
As already pointed out in Fig. 1, a catalytic process with low reaction time is desirable to achieve an efficient methodology for glycerol carbonate production. Since most of the procedures found over literature reports temperatures above 80 °C, the use of microwave irradiation can be a good alternative to enhance reaction kinetics and reduce reaction time.3–5 Unfortunately, microwave irradiation protocols are not scalable to the extent need for glycerol carbonate production but Kappe and co-workers have shown recently that such procedures can be easily translated to continuous-flow protocols where scalability is not a problem.6
Different groups have attempted to use ionic liquids7–14 and lipases15–20 as catalysts to produce glycerol carbonate with high yield and selectivity but this strategies are inadequate in terms of high reaction times and prices of the catalysts used, sometimes not commercially available. Other catalysts such as gold,21 palladium,22,23 copper,24–26 rhodium,27 tungsten,28,29 zinc,10,30 lanthanum,31–33 calcium,34,35 magnesium,36–41 zeolites,42,43 Nafion,44 alumina,45–47 have been used to produce glycerol carbonate by carbonylation of glycerol with alkyl carbonates,19,48,49 CO2 (ref. 30) or urea,50–55 arriving on the desired product with poor (when CO2 is used) to good yields and selectivity (when alkyl carbonates are used). A more convergent approach tends to arrive on the desired glycerol carbonate during the biodiesel process and good results were obtained up to now (Fig. 2).17,56–61
Besides all catalysts already used on the glycerol carbonate production, nucleophilic catalysis performed by tertiary amines seems to be the most reliable procedure since they are safe, easy to separate from reaction media and cheap, where DABCO,62 DBU,12,63 TEA64 and imidazole65 are the most used.
Here in we report our effort on optimizing glycerol carbonate production catalyzed by several tertiary amines, under microwave irradiation and translating the best reaction conditions to continuous-flow protocol aiming to obtain better productivities.
2. Materials and methods
2.1 Materials
Glycerol (glycerin), purchased from Vetec Chemistry, dimethyl carbonate (DMC), glyceryl carbonate, the ionic liquid [BMIM][AcO] and all of the amines used: N-methylimidazole, imidazole, DABCO (1,4-diazabicyclo [2,2,2] octane), immobilized DABCO hydrochloride (1,4-diazabicyclo [2,2,2] octane), DMAP (4-dimethylaminopyridine), DBU (1,8-diazibiciclo [5.4.0] undec-7-ene), HMTA (hexamethylenetetramine), triethylamine and pyridine, were purchased from Sigma-Aldrich. All other materials were at least reagent-grade.2.2 Batch reactions
Glycerol (3 mmol, 1 eq.), DMC (9 mmol, 3 eq.) and a catalyst (sodium acetate, [BMIM][AcO] and N-methylimidazole) (0.23 mmol, 7.7 mol%) were added to 4 mL vials on silicon carbide plates. Reactions were performed at 90 °C for 2 h. Conversions were analyzed as described in Section 2.5.72.3 Microwave reactions
Microwave irradiation experiments were carried out using a Monowave 300 single-mode microwave reactor from Anton Paar GmbH (Graz, Austria). The experiments were performed in a 10 mL Pyrex microwave process vial equipped with a magnetic stirring bar at a rate of 600 rpm. Reaction times refer to hold times at the temperatures indicated and not to total irradiation times. The reaction conversions were evaluated by GCMS (item 2.5).2.4 Continuous-flow reactions
The GC synthesis conditions by microwave irradiation were also performed under continuous flow conditions. For this purpose, the same reactional conditions (molar ratio between glycerol/DMC and temperature) were maintained. Catalyst concentrations of 0.1, 0.5, 1.5 and 2.5 mol% were investigated once it was possible to reduce it under microwave irradiation. Glycerol and DBU were mixed and previously heated to 70 °C to turned the mixture able to de pumped by the first pump (solution A). Flows of each pump from Syrris Asia system were carefully adjusted to maintain the proportion of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between the flows of pump A and B. Pump B (DMC) worked with a flow 3 times higher than pump A (glycerol + DBU), and for this reason, both solution were pumped together with 1 or 2 mL min−1. When pump A worked with 0.234 mL min−1 and pump B with 0.766 mL min−1, the final solution was mixture in a tubular reactor of 16 mL (1/16 i.d.), with a final flow rate of 1 mL min−1, resulting in a reaction time of 16 min. The isolation of the desired product was performed in a distillation system Buchi B-585 Glass oven (bulb-to-bulb distillation).
1 between the flows of pump A and B. Pump B (DMC) worked with a flow 3 times higher than pump A (glycerol + DBU), and for this reason, both solution were pumped together with 1 or 2 mL min−1. When pump A worked with 0.234 mL min−1 and pump B with 0.766 mL min−1, the final solution was mixture in a tubular reactor of 16 mL (1/16 i.d.), with a final flow rate of 1 mL min−1, resulting in a reaction time of 16 min. The isolation of the desired product was performed in a distillation system Buchi B-585 Glass oven (bulb-to-bulb distillation).
2.5 GC analysis
The GC-MS analysis was performed by using a modified method from ref. 20. Glyceryl carbonate and derived from the reactions were transformed into more volatile silylated derivatives in the presence of N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA). All GC-MS measurements were carried out in triplicate using a DB 5 (Agilent, J&W. Scientific®, USA) capillary column (30 m × 0.25 mm × 0.25 μm). The GC-MS samples were prepared by dissolving 10 μL of the final product in 1 mL of chloroform. 1 μL of this sample was then injected into Shimadzu CG2010 equipment. The injector and detector temperatures were 250 °C, and the oven temperature was constant at 60 °C for 1 min, and then increased by 10 °C min−1 to 250 °C, where it was held constant for 3 min. The percentages of conversion and selectivity were analyzed by the area of the chromatograms. GC-FID:HP-5MS capillary column (5% phenyl methyl polysiloxane capillary, 30.0 m × 250 μm × 250 μm). Helium was used as carrier gas. 1 μL samples were injected at 100 °C. The oven was heated at 15 °C min−1 to 150 °C, at 8 °C min−1 to 200 °C, at 2 °C min−1 to 240 °C, and then maintained for 4 min. After this, the oven was heated at 15 °C min−1 to 300 °C.3. Results and discussion
In the beginning of our studies, our group was first curious (intrigued) about the use of ionic liquids as catalyst for glycerol carbonate production, already reported by several authors over literature.7,8,10–14,62,66 Some authors have already proposed that the catalytic activity of some ionic liquids in this reaction can be related to the basicity of the counter anion however we postulated that ionic liquids contaminated with small amounts of methyl imidazole could also catalyze this reaction as suggested above. If it was true it could be further questioned: why use an expensive catalyst, like ionic liquids, to produce glycerol carbonate?To evaluate the use of ionic liquids in catalyzing the synthesis of glycerol carbonate, we used standard conditions establish in literature. After screening different ionic liquids, [BMIM][AcO] appeared as the most promising and was further evaluated. Glycerol and DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) were mixed together and reacted for 2 hours at 90 °C using 7.7% mol of [BMIM][AcO]. Besides ionic liquid, we also decided to evaluate the methylimidole percursor, once it is reported that it is a known contaminant in different IL. The results are shown in Table 1.
3) were mixed together and reacted for 2 hours at 90 °C using 7.7% mol of [BMIM][AcO]. Besides ionic liquid, we also decided to evaluate the methylimidole percursor, once it is reported that it is a known contaminant in different IL. The results are shown in Table 1.
| Entry | Catalyst | Conv. (%) | Selectivity (%) |
|---|---|---|---|
a Reaction conditions: catalystic (7.7 mol%), glycerol and DMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 mmol), 90 °C, 2 h. Conventional heating. 9 mmol), 90 °C, 2 h. Conventional heating. |
|||
| 1 | [BMIM][AcO] | 81 | 81 |
| 2 | Methyl imidazol | 85 | 85 |
As shown in Table 1, results obtained with [BMIM][AcO] ionic liquid are similar to those obtained when methyl imidazole.
We decided to look more carefully to the reaction catalyzed by methyl imidazole (MIM) and further evaluate different reaction parameters in order to optimize the reaction conditions, particularly the use of continuous-flow. Now, microwave irradiation was used as a standard heating technique in order to have reaction conditions that could be easily translated to continuous-flow process.6 Temperature, glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC molar ratio and reaction time were screened (see ESI† for further details) and it was found that 120 °C, 1
DMC molar ratio and reaction time were screened (see ESI† for further details) and it was found that 120 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 30 minutes lead to the best conversions and selectivity's towards the desired product. The amount of catalyst was also investigated and the results are shown in Table 2.
3 and 30 minutes lead to the best conversions and selectivity's towards the desired product. The amount of catalyst was also investigated and the results are shown in Table 2.
| Entry | X mol% MIM | Conv. (%) | Selectivity (%) |
|---|---|---|---|
a Reaction conditions: glycerol and DMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 mmol), 120 °C, 30 min, MW. 9 mmol), 120 °C, 30 min, MW. |
|||
| 1 | 0 | 0 | 0 |
| 2 | 0.5 | 37 | 90 |
| 3 | 1 | 57 | 84 |
| 4 | 2.5 | 83 | 83 |
| 5 | 5 | 85 | 79 |
| 6 | 7.7 | 85 | 85 |
Table 2 presents the results obtained for different methyl imidazole percentage on glycerol carbonate production under microwave irradiation. It is possible to note that from 2.5 to 7.7 mol% of catalyst (entries 4–6, Table 2) just a small increase on conversion is obtained and do not justify the use of higher amounts of catalyst.
With these results in hands we decided to explore the potential of the developed methodology to other amines such as DMAP, DBU, DABCO, HMTA, TEA and pyridine. The results are presented on Table 3.
| Catalysts | Conv. (%) | Sel. (%) |
|---|---|---|
a Reaction conditions: catalysts (2.5 mol%), glycerol and DMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 mmol), 120 °C, 30 min. 9 mmol), 120 °C, 30 min. |
||
| N-Methylimidazole | 83 | 85 |
| DMAP | 86 | 76 |
| DBU | 85 | 78 |
| DABCO | 86 | 75 |
| HTMA | 80 | 76 |
| TEA | 88 | 75 |
| Pyridine | 88 | 78 |
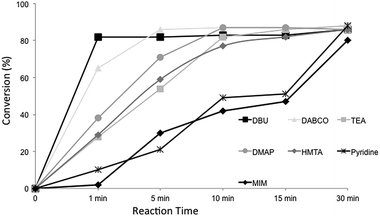
A first look at Table 3 shows that all catalysts present similar behavior achieving comparable conversion for glycerol carbonate after 30 minutes of reaction, without loss of selectivity. But the evolution of glycerol carbonate formation during the 30 minutes is totally different depending on the catalyst chosen. Fig. 1 shows the values of conversion at different reaction times for the catalysts mentioned above.
As presented here, very good conversion could be obtained for DBU after just one minute of reaction under microwave irradiation. DABCO was also good and lead to high conversions after 5 minutes. The other nucleophilic catalysts have shown also good performance but just after 10 minutes of reaction. When we turn the results obtained in Fig. 1 into productivity of each catalyst, DBU pop-ups from the others, presenting high productivities due to the very fast reaction (Table 4). On the other hand, methyl imidazole (MIM) presented very low productivity when compared to the other nucleophilic catalysts.
| Catalyst | Productivitya | USD/mmol |
|---|---|---|
| a Productivity: (g of product per h per mmol of catalyst) reaction conditions: glycerol (0.92 g, 10 mmol), DMC (2.67 g, 30 mmol), temp: 120 °C (MW), catalyst: 0.025 mmol% (w.r.t glycerol). | ||
| DMAP | 14.42 | 1.00 |
| MIM | 5.23 | 0.053 |
| DBU | 153.88 | 0.38 |
| DABCO | 31.90 | 0.19 |
| HTMA | 8.94 | 0.05 |
| TEA | 10.63 | 0.026 |
| Pyridine | 5.04 | 0.09 |
The same heating profile cannot be properly performed under batch conditions using reflux condenser since dimethyl carbonate boiling point is around 90 °C, which drops the conversion of the reaction to 50% when catalyzed by DBU.
The use of microwave irradiation can take chemistry to new process windows but scaling up the conditions already optimized is still a challenge. As proposed by Kappe and co-workers this conditions can be easily translated to continuous-flow protocols where conventional heating can take place as fast as inside the microwave reactor due to the high surface to volume ratio.
With these results in hands we start to develop our continuous-flow process. Fa T-piece (4or this purpose we have used to syringe pumps connected through mL mixing zone) into a 16 mL coil (heated at 120 °C) and 100 psi back pressure regulator, as shown in Fig. 3. A solution of DBU in Glycerol was pumped through pump A (0.234 mL min−1) and mixed with neat DMC from pump B (0.766 mL min−1) at a total flow rate of 1 mL min−1, respecting the stoichiometry optimized under microwave irradiation, leading to a residence time of 16 minutes.
 | ||
| Fig. 3 Typical conversion time profile. Reaction conditions: glycerol (0.92 g, 10 mmol), DMC (2.67 g, 30 mmol), temp: 120 °C (MW), catalyst: 0.025 mmol% (w.r.t glycerol). | ||
The set up presented on Fig. 4 lead to 84% of conversion and 86% of selectivity to the desired glycerol carbonate. Further optimization was also performed trying to reduce residence time and the amount of DBU used as catalyst. Under a total flow rate of 2 mL min−1 (0.468 mL min−1 – pump A and 1.532 mL min−1 pump B) and using 1.5% mol of DBU, 80% of conversion could be obtained with 82% of selectivity with chemical yield isolated 79% to glycerol carbonate. The system was stable for 5 hours of operation where now change on conversion and selectivity was observed.
As already reported by other authors61,67 glycerol carbonate can be obtained direct from vegetable oils through a cascade reaction where glycerol from triacylglycerol hydrolysis is used in situ for the reaction with DMC and glycerol carbonate formation. We were also interested in this approach and decided to test the protocol optimized with DBU under continuous-flow conditions. The results suggest that catalysis by DBU promotes coproduction of biodiesel and glycerol carbonate under continuous-flow conditions (Table 5).
| Entry | Flow rate (mL min−1) | Residence time (min) | Glycerol carbonate (%) | Biodiesel (%) |
|---|---|---|---|---|
| 1 | 1 | 16 | 90 | 80 |
| 2 | 2 | 8 | 70 | 62 |
4. Conclusion
In conclusion we were able to develop a protocol for a tertiary amine catalyzed glycerol carbonate production using microwave irradiation and its translation into continuous-flow system. With all the catalysts tested, low catalyst loading, high yields and selectivity were observed in only 30 minutes under microwave irradiation. In addition, among the catalysts tested DBU presented higher productivity and its reaction translated into continuous-flow. Under solventless continuous-flow, 80% yield and 82% selectivity was observed in 16 minutes residence time. These conditions lead to a space-time yield of 24 kg per L per day for the production of glycerol carbonate. The same continuous-flow system was also used to obtain glycerol carbonate direct from vegetable oil with 80.0% biodiesel production and 90.0% production of glycerol carbonate was reached.Acknowledgements
Authors thanks CAPES, CNPq, FAPERJ for financial support.References
- http://www.grandviewresearch.com/press-release/global-glycerol-market.
- J. R. Ochoa-Gomez, O. Gomez-Jimenez-Aberasturi, C. Ramirez-Lopez and M. Belsue, Org. Process Res. Dev., 2012, 16, 389–399 CrossRef CAS.
- C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS PubMed.
- C. O. Kappe, Chem. Soc. Rev., 2008, 37, 1127–1139 RSC.
- C. O. Kappe, B. Pieber and D. Dallinger, Angew. Chem., Int. Ed., 2012, 52, 1088–1094 CrossRef PubMed.
- T. N. Glasnov and C. O. Kappe, Chem.–Eur. J., 2011, 17, 11956–11968 CrossRef CAS PubMed.
- C. Chiappe and S. Rajamani, Pure Appl. Chem., 2012, 84, 755–762 CAS.
- H.-J. Cho, H.-M. Kwon, J. Tharun and D.-W. Park, J. Ind. Eng. Chem., 2010, 16, 679–683 CrossRef CAS.
- J. S. Choi, F. S. H. Simanjuntaka, J. Y. Oh, K. I. Lee, S. D. Lee, M. Cheong, H. S. Kim and H. Lee, J. Catal., 2013, 297, 248–255 CrossRef CAS.
- D.-W. Kim, M.-J. Kim, K. Roshith, M.-I. Kim, J.-Y. Kwak and D.-W. Park, Korean J. Chem. Eng., 2014, 31, 972–980 CrossRef CAS.
- D.-W. Kim, K.-A. Park, M.-J. Kim, D.-H. Kang, J.-G. Yang and D.-W. Park, Appl. Catal., A, 2014, 473, 31–40 CrossRef CAS.
- M. K. Munshi, P. S. Biradar, S. M. Gade, V. H. Rane and A. A. Kelkar, RSC Adv., 2014, 4, 17124–17128 RSC.
- P. D. Won, Korean Chem. Eng. Res., 2013, 51, 347–351 CrossRef.
- Y. Yi, Y. Shen, J. Sun, B. Wang, F. Xu and R. Sun, Chin. J. Catal., 2014, 35, 757–762 CrossRef CAS.
- S. C. Kim, Y. H. Kim, H. Lee, D. Y. Yoon and B. K. Song, J. Mol. Catal. B: Enzym., 2007, 49, 75–78 CrossRef CAS.
- K. H. Lee, C.-H. Park and E. Y. Lee, Bioprocess Biosyst. Eng., 2010, 33, 1059–1065 CrossRef CAS PubMed.
- J. Y. Min and E. Y. Lee, Biotechnol. Lett., 2011, 33, 1789–1796 CrossRef CAS PubMed.
- G. Ou, B. He and Y. Yuan, Enzyme Microb. Technol., 2011, 49, 167–170 CrossRef CAS PubMed.
- M. Tudorache, A. Negoi, L. Protesescu and V. I. Parvulescu, Appl. Catal., B, 2014, 145, 120–125 CrossRef CAS.
- M. Tudorache, L. Protesescu, S. Coman and V. I. Parvulescu, Green Chem., 2012, 14, 478–482 RSC.
- C. Hammond, J. A. Lopez-Sanchez, M. H. Ab Rahim, N. Dimitratos, R. L. Jenkins, A. F. Carley, Q. He, C. J. Kiely, D. W. Knight and G. J. Hutchings, Dalton Trans., 2011, 3927–3937 RSC.
- S. P. Chavan and B. M. Bhanage, Tetrahedron Lett., 2014, 55, 1199–1202 CrossRef CAS.
- J. Hu, J. Li, Y. Gu, Z. Guan, W. Mo, Y. Ni, T. Li and G. Li, Appl. Catal., A, 2010, 386, 188–193 CrossRef CAS.
- M. Casiello, A. Monopoli, P. Cotugno, A. Milella, M. M. Dell'Anna, F. Ciminale and A. Nacci, J. Mol. Catal. A: Chem., 2014, 381, 99–106 CrossRef CAS.
- W. H. Chul, Clean Technology, 2013, 19, 416–422 CrossRef.
- J. Zhang and D. He, J. Colloid Interface Sci., 2014, 419, 31–38 CrossRef CAS PubMed.
- N. N. Ezhova, I. G. Korosteleva, N. V. Kolesnichenko, A. E. Kuz'min, S. N. Khadzhiev, M. A. Vasil'eva and Z. D. Voronina, Pet. Chem., 2012, 52, 91–96 CrossRef CAS.
- K. Jagadeeswaraiah, C. R. Kumar, P. S. S. Prasad and N. Lingaiah, Catal. Sci. Technol., 2014, 4, 2969–2977 CAS.
- K. Jagadeeswaraiah, C. R. Kumar, P. S. S. Prasad, S. Loridant and N. Lingaiah, Appl. Catal., A, 2014, 469, 165–172 CrossRef CAS.
- H. Li, D. Gao, P. Gao, F. Wang, N. Zhao, F. Xiao, W. Wei and Y. Sun, Catal. Sci. Technol., 2013, 3, 2801–2809 CAS.
- S. R. Lim, S. D. Lee, H. S. Kim, F. S. H. Simanjuntak and H. Lee, Bull. Korean Chem. Soc., 2014, 35, 3163–3168 CrossRef CAS.
- F. S. H. Simanjuntak, V. T. Widyaya, C. S. Kim, B. S. Ahn, Y. J. Kim and H. Lee, Chem. Eng. Sci., 2013, 94, 265–270 CrossRef CAS.
- L. Wang, Y. Ma, Y. Wang, S. Liu and Y. Deng, Catal. Commun., 2011, 12, 1458–1462 CrossRef CAS.
- P. Lu, H. Wang and K. Hu, Chem. Eng. J., 2013, 228, 147–154 CrossRef CAS.
- F. S. H. Simanjuntak, T. K. Kim, S. D. Lee, B. S. Ahn, H. S. Kim and H. Lee, Appl. Catal., A, 2011, 401, 220–225 CrossRef CAS.
- M. Du, Q. Li, W. Dong, T. Geng and Y. Jiang, Res. Chem. Intermed., 2012, 38, 1069–1077 CrossRef CAS.
- P. Liu, M. Derchi and E. J. M. Hensen, Appl. Catal., A, 2013, 467, 124–131 CrossRef CAS.
- M. Malyaadri, K. Jagadeeswaraiah, P. S. S. Prasad and N. Lingaiah, Appl. Catal., A, 2011, 401, 153–157 CrossRef CAS.
- G. Parameswaram, M. Srinivas, B. H. Babu, P. S. S. Prasad and N. Lingaiah, Catal. Sci. Technol., 2013, 3, 3242–3249 CAS.
- F. S. H. Simanjuntak, S. R. Lim, B. S. Ahn, H. S. Kim and H. Lee, Appl. Catal., A, 2014, 484, 33–38 CrossRef CAS.
- L. Zheng, S. Xia, Z. Hou, M. Zhang and Z. Hou, Chin. J. Catal., 2014, 35, 310–318 CrossRef CAS.
- Y. T. Algoufi and B. H. Hameed, Fuel Process. Technol., 2014, 126, 5–11 CrossRef CAS.
- S. Pan, L. Zheng, R. Nie, S. Xia, P. Chen and Z. Hou, Chin. J. Catal., 2012, 33, 1772–1777 CrossRef CAS.
- M. J. Climent, A. Corma, S. Iborra, S. Martinez-Silvestre and A. Velty, ChemSusChem, 2013, 6, 1224–1234 CrossRef CAS PubMed.
- R. Bai, Y. Wang, S. Wang, F. Mei, T. Li and G. Li, Fuel Process. Technol., 2013, 106, 209–214 CrossRef CAS.
- Z. Liu, J. Wang, M. Kang, N. Yin, X. Wang, Y. Tan and Y. Zhu, J. Braz. Chem. Soc., 2014, 25, 152–160 CAS.
- S. Sandesh, G. V. Shanbhag and A. B. Halgeri, Catal. Lett., 2013, 143, 1226–1234 CrossRef CAS.
- M. Selva, V. Benedet and M. Fabris, Green Chem., 2012, 14, 188–200 RSC.
- A. Takagaki, K. Iwatani, S. Nishimura and K. Ebitani, Green Chem., 2010, 12, 578–581 RSC.
- M. Aresta, A. Dibenedetto, F. Nocito and C. Ferragina, J. Catal., 2009, 268, 106–114 CrossRef CAS.
- M. J. Climent, A. Corma, P. De Frutos, S. Iborra, M. Noy, A. Velty and P. Concepcion, J. Catal., 2010, 269, 140–149 CrossRef CAS.
- S.-I. Fujita, Y. Yamanishi and M. Arai, J. Catal., 2013, 297, 137–141 CrossRef CAS.
- S.-D. Lee, G.-A. Park, D.-W. Kim and D.-W. Park, J. Nanosci. Nanotechnol., 2014, 14, 4551–4556 CrossRef CAS PubMed.
- Y. Sun, X. Tong, Z. Wu, J. Liu, Y. Yan and S. Xue, Energy Technol., 2014, 2, 263–268 CrossRef CAS.
- T. W. Turney, A. Patti, W. Gates, U. Shaheen and S. Kulasegaram, Green Chem., 2013, 15, 1925–1931 RSC.
- F. A. Dawodu, O. O. Ayodele, J. Xin and S. Zhang, Renewable Energy, 2014, 68, 581–587 CrossRef CAS.
- A. R. Go, Y. Lee, Y. H. Kim, S. Park, J. Choi, J. Lee, S. O. Han, S. W. Kim and C. Park, Enzyme Microb. Technol., 2013, 53, 154–158 CrossRef CAS PubMed.
- H. Jung, Y. Lee, D. Kim, S. O. Han, S. W. Kim, J. Lee, Y. H. Kim and C. Park, Enzyme Microb. Technol., 2012, 51, 143–147 CrossRef CAS PubMed.
- T. Kai, G. L. Mak, S. Wada, T. Nakazato, H. Takanashi and Y. Uemura, Bioresour. Technol., 2014, 163, 360–363 CrossRef CAS PubMed.
- E. E. Kwon, H. Yi and Y. J. Jeon, Chemosphere, 2014, 113, 87–92 CrossRef CAS PubMed.
- P.-J. Seong, B. W. Jeon, M. Lee, D. H. Cho, D.-K. Kim, K. S. Jung, S. W. Kim, S. O. Han, Y. H. Kim and C. Park, Enzyme Microb. Technol., 2011, 48, 505–509 CrossRef CAS PubMed.
- M. K. Munshi, S. M. Gade, V. H. Rane and A. A. Kelkar, RSC Adv., 2014, 4, 32127–32133 RSC.
- M. K. Munshi, S. M. Gade, M. V. Mane, D. Mishra, S. Pal, K. Vanka, V. H. Rane and A. A. Kelkar, J. Mol. Catal. A: Chem., 2014, 391, 144–149 CrossRef CAS.
- J. R. Ochoa-Gomez, O. Gomez-Jimenez-Aberasturi, C. Ramirez-Lopez and B. Maestro-Madurga, Green Chem., 2012, 14, 3368–3376 RSC.
- P. U. Naik, L. Petitjean, K. Refes, M. Picquet and L. Plasseraud, Adv. Synth. Catal., 2009, 351, 1753–1756 CrossRef CAS.
- S. M. Gade, M. K. Munshi, B. M. Chherawalla, V. H. Rane and A. A. Kelkar, Catal. Commun., 2012, 27, 184–188 CrossRef CAS.
- J. Young, M. Eun and Y. Lee, Biotechnol. Lett., 2011, 33, 1789–1796 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02117k |
| This journal is © The Royal Society of Chemistry 2015 |