Synthesis and properties poly(arylene ether sulfone)s with pendant hyper-sulfonic acid†
Jinhui Pang,
Sinan Feng,
Haibo Zhang,
Zhenhua Jiang and
Guibin Wang*
Key Laboratory of Super Engineering Plastic of Ministry of Education, Jilin University, Super Engineering Plastics Engineering Research Center of Ministry of Education, Jilin University No. 2699, Qianjin Street, Changchun 130012, P. R. China. E-mail: wgb@jlu.edu.cn; Fax: +86-431-85168886; Tel: +86-431-85168886
First published on 20th April 2015
Abstract
A new class of poly(arylene ether sulfone) with multiple sulfonic acid groups on aromatic side chains (PAES-nS, n = 2 or 3) were prepared from hydroxyphenyl-containing polymer precursors and sulfonated monomer by graft reaction. Those polymers were soluble in the common organic solvents, such as DMAc, DMF, DMSO and NMP, exhibited good thermal stability, the glass transition temperatures ranged from 200 to 240 °C and the 5% weight loss temperatures were higher than 290 °C. Remarkably, all the PAES-nS membranes exhibited high proton conductivity above 10−2 S cm−1 at room temperature, and low swelling ratio below 26% at 80 °C. Compared with Nafion 117, the PAES-3S-40 with high ion exchange capacity (IEC) value (1.89 mequiv. g−1) exhibited higher proton conductivity and appropriate swelling ratio at the same conditions. A combination of good thermal stability, excellent dimensional stability and high proton conductivities indicates these polymers are good candidate materials for proton exchange membrane in fuel cell applications.
1. Introduction
Proton exchange membranes fuel cells (PEMFCs), which convert chemical energy to electrical energy, are regarded as promising future power sources owing to their advantages, such as high efficiency, high energy density, quiet operation, and environmental friendliness.1 In a PEMFC, proton exchange membrane (PEM) is one of the key components, which allows for the proton transport from the anode to the cathode. The current state-of-the-art PEM materials are perfluorinated polymer such as Nafion or Flemion because of their good physical and chemical stability along with high proton conductivity under a wide range of relative humidity at moderate operation temperatures.2 However, they suffer from disadvantages such as high cost, limited operation temperature (≤80 °C), and high methanol crossover restrict their wide applications. Thus aromatic hydrocarbon polymers are investigated as alternative PEM materials.3–5 Among aromatic hydrocarbon polymers, sulfonated poly(arylene ether)s (SPAEs) are considered as promising candidates for PEM materials because of their high thermochemical stability, high mechanical strength, good film-forming ability and low fuel gas (or liquid) cross-over.6–14 Recently, side-chain-style aromatic polymers have attracted attention extensively.15–25 Poly(arylene ether)s with acidic groups on short pendant chains have been suggested as a strategy to improve the micro-phase separation of hydrophilic and hydrophobic domains, and overcome issues existed in the main-chain-type sulfonated polymer membranes (sulfonic acid groups attached directly to their backbone) that the polymer show unfavorable excess water swelling at an intensive water uptake over a critical temperature or sulfonation degree.26Generally, two methods are adopted to synthesize side-chain-style sulfonated poly(arylene ether)s. One is to chemically modify the polymer, which is sometimes called post-modification or graft method.11 The other is to prepare the polymer based on a sulfonated monomer via direct copolymerization.27–34 From the point of view of synthetic procedure, the first method is preferred since the modified polymer is easy achieved, and the second method may require a tedious approach for monomer synthesis. In previous work, we have reported the synthesis of sulfonated poly(arylene ether)s.35–38 with single sulfonic acid on every pendant side chain. The polymers exhibited high proton conductivity and excellent dimensional stability. Based on our research, we suggested that polymer possess of multiple sulfonic acid groups linked in a side chain structure, which improves dimensional stability and proton conductivity of the materials in PEMFC applicable environment.
In this paper, we successfully prepared a class of novel poly(arylene ether sulfone)s with pendant multiple sulfonic acid groups on aromatic side chains by graft method. Obtained polymer membranes exhibited high proton conductivity and good dimensional stability. These were attributed to their reasonable molecular structures that improved hydrophilic/hydrophobic microphase separation morphology.
2. Experimental
2.1. Materials
3-Methoxyaniline was gotten from Tokyo Chemical Industry. 4-Phenol sulfonic acid sodium salt was purchased from Fluka Chemical Industry. 4,4′-Dichlorodiphenyl sulfone (DCDPS), 4,4′-dihydroxyldiphenyl ether (DHDPE) and tetramethylene sulfone (TMS) were obtained from Yanji Chemical Plant, China. 1,4-Bi(4-fluorobenzoyl)benzene, 4,4′-difluorobenzophenone and 1,4-benzoquinone were offered by Dalian Jinzhou Chemical Reagent, China. All the other chemicals were purchased from commercial resources and were purified by conventional method.2.2. Synthesis of monomers
IR (cm−1): 3403 (–OH), 2832 (–OCH3).1H NMR (500 MHz, DMSO-d6, δ): 8.75 (s, 1H), 8.73 (s, 1H), 7.28 (t, J = 8.5 Hz, 1H), 7.05 (m, 2H), 6.84 (m, 1H), 6.73 (d, J = Hz, 1H), 6.66 (d, J = 3.0 Hz, 1H), 6.66 (dd, J = 9.0, 3.0, 1H), 3.77 (s, 3H).
IR (cm−1): 1662 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1592 (C
O), 1592 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1085 (Ar–SO3Na); 1H NMR (500 MHz, DMSO-d6, δ): 8.07 (dd, J = 6.8 Hz, 2.0 Hz, 2H), 7.74 (m, 2H), 7.36 (m, 2H).
C), 1085 (Ar–SO3Na); 1H NMR (500 MHz, DMSO-d6, δ): 8.07 (dd, J = 6.8 Hz, 2.0 Hz, 2H), 7.74 (m, 2H), 7.36 (m, 2H).
1,4-Bi(3-sodium sulfonate-4-fluorobenzoyl) benzene as monomer b was synthesized as described in ref. 41 and 42.
IR (cm−1): 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1211, 1093, 621 (Ar–SO3Na). 1H NMR (500 MHz, DMSO-d6, δ): 8.12–8.15 (dd, J = 2.3 Hz, 4.5 Hz, 2H), 7.87 (s, 4H) 7.81–7.84 (m, 2H), 7.34–7.38 (dd, J = 5.3 Hz, 5.3 Hz, 2H).
O), 1211, 1093, 621 (Ar–SO3Na). 1H NMR (500 MHz, DMSO-d6, δ): 8.12–8.15 (dd, J = 2.3 Hz, 4.5 Hz, 2H), 7.87 (s, 4H) 7.81–7.84 (m, 2H), 7.34–7.38 (dd, J = 5.3 Hz, 5.3 Hz, 2H).
2.3. Synthesis of polymers
The PAES-2S-b polymers were prepared using the same synthesis and purification routine as PAES-2S-a. The only difference was that sodium 5,5′-carbonylbis(2-fluorobenzenesulfonate) was replaced by 1,4-bi(3-sodium sulfonate-4-fluorobenzoyl) benzene. Yield: ∼92%.
2.4. Polymer analysis and measurements
The viscosities of the obtained polymers were determined using Ubbelohde viscometer in thermostatic container with the polymer concentration of 0.5 g dL−1 in NMP at 25 °C. FT-IR spectra were measured on a Nicolet Impact 410 Fourier-transform infrared spectrometer. 1H NMR experiments were carried out on a Bruker 510 spectrometer (1H, 500 MHz and 13C, 125 MHz) using DMSO-d6 as solvent. Gel permeation chromatography (GPC) was performed on a Polymer Laboratories PL-GPC 220 instrument using DMF as the eluent at 80 °C. The obtained molecular weight is relative to the polystyrene standard.The water uptake content was calculated by
| Water uptake (%) = [(Wwet − Wdry)/Wdry] × 100% | (1) |
Dimensional change of the polymer membranes was investigated by immersing the sample films in deionized water to reach equilibrium at desired temperature.
The change of film thickness and length were calculated from
| ΔT = [(Twet − Tdry)/Tdry] × 100%, ΔL = [(Lwet − Ldry)/Ldry] × 100% | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ, and domain periodicities (D) were calculated from Gaussian fits to the principal scattering maxima of the Lorentz-corrected intensities using D = 2πq−1.
θ/λ, and domain periodicities (D) were calculated from Gaussian fits to the principal scattering maxima of the Lorentz-corrected intensities using D = 2πq−1.3. Results and discussion
3.1. Synthesis of polymers
According to literature report,44 the hydrophilic domains of sulfonated aromatic polymers were primarily responsible for water absorption. Typically, the equilibrium water absorption of disulfonated poly(arylene ether sulfone)s, increases linearly up to about 0.44 mole fraction of disulfonated units. Beyond this level of disulfonation, water uptake increases drastically, signifying a change in phase morphology45,46 and decline in dimensional stability, which results in loss of mechanical performance. Polymer with sulfonated side chain structure can improve water management at high ion exchange capacity (IEC) level. Therefore we designed and prepared a series of multiple sulfonated side chain PAES with high IEC close to 2.0 mequiv. g−1. Firstly, PAES containing 0.4, 0.5 and 0.6 molar percentage of pendant methoxyphenyl group (PAES-OCH3-40, -50 and -60) were prepared by nucleophilic polycondensation reaction using 0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, 0.5
0.4, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 0.4
0.5 and 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6 as feed ratios of DHDPE/MeOPHQ (Scheme 1). The polymerization reaction proceeded smoothly, and no evident crosslinking was observed when the system was carefully purged with nitrogen and the temperature controlled by oil bath as well. As shown in Table S1,† GPC results showed that polymer with high molecular weight (Mn > 90
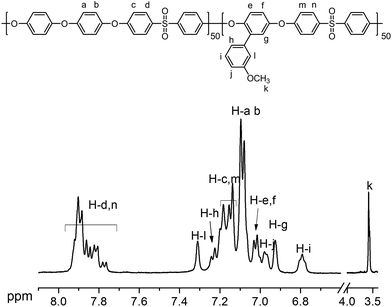
0.6 as feed ratios of DHDPE/MeOPHQ (Scheme 1). The polymerization reaction proceeded smoothly, and no evident crosslinking was observed when the system was carefully purged with nitrogen and the temperature controlled by oil bath as well. As shown in Table S1,† GPC results showed that polymer with high molecular weight (Mn > 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was obtained and the polydispersity (PD) ranged from 1.8 to 2.3. The 1H NMR spectrum of PAES-OCH3-50 is shown in Fig. 1. The signal of H atom in methoxyl group appeared at low chemical shift and the signal of H atom in meta-position of –SO2– groups appeared at high chemical shift.
000 g mol−1) was obtained and the polydispersity (PD) ranged from 1.8 to 2.3. The 1H NMR spectrum of PAES-OCH3-50 is shown in Fig. 1. The signal of H atom in methoxyl group appeared at low chemical shift and the signal of H atom in meta-position of –SO2– groups appeared at high chemical shift.
Then a series of PAES-OH was obtained by dimethyl reaction of PAES-OCH3 in pyridine hydrochloride (Scheme 2). It was found that the methoxyl group could be converted completely to hydroxyl group in excess molten pyridine hydrochloride after 8 h. GPC data (in Table S1†) confirmed that PAES-OH polymer was achieved without degradation after dimethyl reaction. Compared to PAES-OCH3, the PD of PAES-OH slightly increased ranging from 1.8 to 2.5. In their 1H NMR spectra (Fig. 2), the peak at 3.7 ppm corresponding to H atom of –OCH3 disappeared completely and new peak appeared at around 9.5 ppm (assigned H atom of phenol hydroxyl), which confirmed the success of complete dimethyl reaction.
The disulfonated poly(arylene ether sulfone)s (PAES-2S) were prepared by PAES-OH and excess disulfonated monomer a or b by nucleophilic substitution reaction (Scheme 2). To eliminate the occurrence of cross-linking reaction, the feed molar ratio of fluorine atom (in disulfonated monomer) and hydroxyl group (in PAES-OH) was higher than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The disulfonated monomer was expected to react with merely one hydroxyl group thus acting as the graft monomer in this reaction. As shown in Fig. 3, 1H NMR study showed that the complete disappearance of the proton of the –OH group at 9.5 ppm was observed, and the proton H-m, H-p of PAES-2S-a50 polymer located in ortho position of sulfonic acid groups appeared at 8.15–8.05 and 8.25–8.18 ppm, respectively, due to the influence of electron withdrawing fluorine atom. Compared with the polymers before graft, all grafted sulfonated polymer showed obvious characteristic absorption band in FT-IR spectra. The peaks at 1658 cm−1 were assigned to stretching vibration band of –C
1. The disulfonated monomer was expected to react with merely one hydroxyl group thus acting as the graft monomer in this reaction. As shown in Fig. 3, 1H NMR study showed that the complete disappearance of the proton of the –OH group at 9.5 ppm was observed, and the proton H-m, H-p of PAES-2S-a50 polymer located in ortho position of sulfonic acid groups appeared at 8.15–8.05 and 8.25–8.18 ppm, respectively, due to the influence of electron withdrawing fluorine atom. Compared with the polymers before graft, all grafted sulfonated polymer showed obvious characteristic absorption band in FT-IR spectra. The peaks at 1658 cm−1 were assigned to stretching vibration band of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in side chain. The asymmetric and symmetric stretching bands of sulfonic acid groups were at 1087 cm−1 and 1029 cm−1, respectively. For example, Fig. 4 shows the FT-IR spectra of PAES-2S-a50 and PAES-2S-b50. 13C NMR spectra of disulfonated polymer (PAES-2S-a50 and PAES-2S-b50) are also given in Fig. 5. The signal at around 193.9 ppm was assigned to the carbonyl carbon atom of side chain. The peak at around 140 ppm could be assigned to the carbon atom attaching –SO3Na group. The above result further confirmed structure of the disulfonated polymer.
O in side chain. The asymmetric and symmetric stretching bands of sulfonic acid groups were at 1087 cm−1 and 1029 cm−1, respectively. For example, Fig. 4 shows the FT-IR spectra of PAES-2S-a50 and PAES-2S-b50. 13C NMR spectra of disulfonated polymer (PAES-2S-a50 and PAES-2S-b50) are also given in Fig. 5. The signal at around 193.9 ppm was assigned to the carbonyl carbon atom of side chain. The peak at around 140 ppm could be assigned to the carbon atom attaching –SO3Na group. The above result further confirmed structure of the disulfonated polymer.
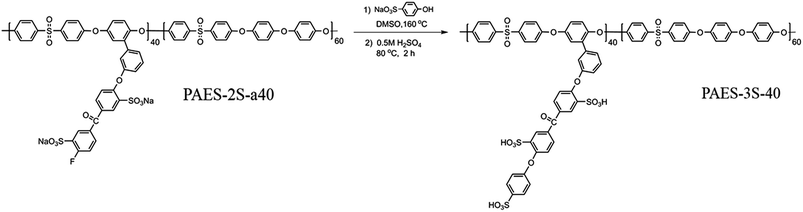
The trisulfonated poly(arylene ether sulfone) (PAES-3S-40) was prepared from PAES-2S-a40 and 4-phenol sulfonic acid sodium salt by nucleophilic substitution reaction (Scheme 3). The structure of PAES-3S-40 was observed by 1H NMR spectrum (Fig. 3). Compared with the disulfonated polymer, the signal at 8.15–8.05 ppm completely disappeared, because electron withdrawing fluorine atom was substituted. All polymers were soluble in polar organic solvents, and their solubility behavior is shown in Table 1. Transparent, flexible, and tough membranes could be formed after solution casting. Intrinsic viscosity values of all sulfonated polymers were higher than 1.60 dL g−1 in NMP at 25 °C, which is much higher than the polymer precursors (PAES-OH-X and PAES-OCH3-X). Admittedly, the high viscosity of sulfonated polymers can be attributed to the increase of molecular weight after the graft reaction. However, it is the enhancement of interchain interactions caused by sulfonic acid groups that should take more responsibility for the increase in viscosity.
| Polymer | ηinh (dL g−1) | Solubility | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X = 40 | X = 50 | X = 60 | NMP | DMSO | DMAc | DMF | THF | Acetone | Water | |
| a Key: (++) soluble at room temperature; (+−) soluble by heating; (−−) insoluble. | ||||||||||
| PAES-2S-aX | 1.72 | 1.88 | 2.10 | ++a | ++ | ++ | +− | −− | −− | −− |
| PAES-2S-bX | 1.65 | 1.80 | 1.99 | ++ | ++ | ++ | +− | −− | −− | −− |
| PAES-3S-X | 2.08 | — | — | ++ | ++ | ++ | +− | −− | −− | −− |
| PAES-OH-X | 0.88 | 0.92 | 0.99 | ++ | ++ | ++ | ++ | +− | −− | −− |
| PAES-OCH3-X | 0.66 | 0.70 | 0.72 | ++ | ++ | ++ | ++ | ++ | −− | −− |
3.2. Thermal properties
Thermal analyses for the polymers were carried out as summarized in Table 2. All the sulfonated polymers had high Tg values ranged from 200 to 240 °C. Interestingly, as the content of pendant group increased, the Tg values of the polymers with methoxy pendant group showed a linear decrease appreciably, while the Tg of the ones with hydroxyl and sulfonic acid pendant group had a linear increase (Fig. 6). As see in Table 2, the Tg value of PAES-OH-60 was 27 °C higher than PAES-OCH3-60, and ones of PAES-2S-b60 and PAES-2S-a60 were higher than PAES-OCH3-60 up to 50 °C and 63 °C, respectively. Compared with methoxy group, the higher polar hydroxyl and sulfonic acid groups can form stronger intermolecular interactions. In addition, the grafted polymer possess bigger pendant group than methoxy polymer to offer a stronger steric hindrance effect. In each of the series, the Tg value increased with the increasing content of sulfonic acid groups (IEC). The thermal stability was evaluated by TGA. As shown in Table 2, all the sulfonated polymers exhibited high onset weight loss (>260 °C) and 5% weight loss (>295 °C) temperatures. Thermal stability of PASE-2S-b was higher than that of PAES-2S-a, since PASE-2S-b contained less sulfonic acid groups than PAES-2S-a. The TGA curve are summarized in Fig. 7, the PAES-2S membranes exhibited a typical two-step degradation pattern. The first weight loss in the range of 260–400 °C was attributed to sulfonic acid group degradation, and the second stage weight loss around 500 °C was assigned to the decomposition of polymer main chain.| Polymer (PAES-nS) | Thermal stability | Oxidative stability | Dimensional stability | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tdonset (°C) | Td5% (°C) | RW (%) | t (h) | 20 °C | 80 °C | |||
| ΔT% | ΔL% | ΔT% | ΔL% | ||||||
| PAES-2S-a60 | 227 | 265 | 297 | 97.2 | >10 | 18.4 | 16.2 | 24.0 | 22.0 |
| PAES-2S-a50 | 224 | 296 | 339 | 98.4 | >10 | 14.2 | 10.0 | 15.7 | 11.1 |
| PAES-2S-a40 | 210 | 309 | 457 | 99.0 | >10 | 6.6 | 6.0 | 9.2 | 8.9 |
| PAES-2S-b60 | 219 | 290 | 310 | 98.0 | >10 | 16.0 | 13.2 | 22.1 | 20.0 |
| PAES-2S-b50 | 216 | 300 | 342 | 99.4 | >10 | 7.1 | 6.8 | 14.2 | 10.0 |
| PAES-2S-b40 | 206 | 314 | 459 | 99.5 | >10 | 2.2 | 2.0 | 4.5 | 4.1 |
| PAES-3S-40 | 235 | 270 | 300 | 96.0 | >10 | 19.5 | 18.4 | 25.6 | 23.2 |
 | ||
| Fig. 6 Relationship between Tg and pendant group content (X), Tg of PEEES (made by homopolymerization of DHDPE with DCDPS) is 176 °C. | ||
3.3. Oxidative stability
As shown in Table 2, all sulfonated membranes showed good oxidative resistance due to their wholly aromatic structure. In addition, the tailored side-chain structure was also profitable for oxidative stability since sulfonic acid groups located only at pendant side chains and the effect of sulfonic acid groups on the hydrolysis of main chain was greatly minimized accordingly. The retained weights of the membranes were above 98% after treatment in Fenton's reagent at 80 °C for 1 h. The time that the membranes completely dissolved (>10 h) was longer than those polymers based on SPAE containing flexible aliphatic side chain.36,373.4. Water uptake and dimensional stability
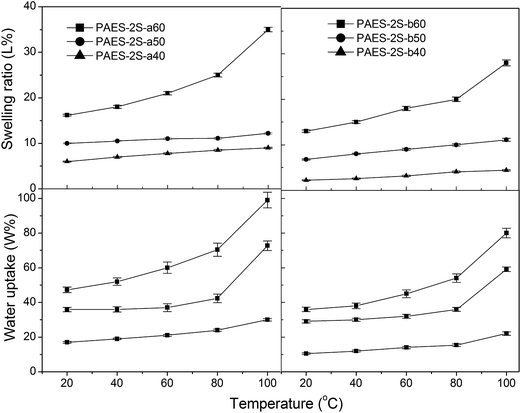
Water management within the membrane is a critical factor in the performance of PEM materials. Water is the main vehicle by which protons are transported through the membrane.47 Therefore, desired water content is an essential requirement for promoting proton conductivity. However, excessively high levels of water in the membrane can result in excessive dimensional changes (swelling) leading to failures in mechanical properties and, in extreme cases, membrane solubility in water. Water uptake is typically a function of the ion exchange capacity (IEC). The polymer PAES-nS was designed to carry reasonable sulfonic acid groups with IEC close to 2.0 mequiv. g−1 which was measured by titration method (Table 3). Fig. 8 clearly shows that the water uptake and swelling ratio continuously increase with IEC and temperature. Below 80 °C, water uptake and swelling ratio of PAES-nS membranes exhibited low dependence on temperature. At 80 °C the highest water uptake and swelling ratio of PAES-2S-a60 were only 70.4% and 22.0%, respectively. Particularly, that trisulfonated polymer film (SPAES-3S-40) owned swelling ratio data similar to Nafion 117 of 20.2%. Above 80 °C, the membranes exhibited a fast increase in water sorption attributed to the formation of large and continuous ion network in the sulfonated polymers. From dimensional data (Table 2), all graft polymers (PAES-nS) had less in-plane direction swelling ratio than through-plane direction. For example, at 80 °C in-plane direction swelling ratio of PAES-2S-a50 was only 11.1% and through-plane direction was 15.7%. According to literature report,48 that is much larger swelling in through-plane direction than in-plane direction. It would be plausible that large (or long) aromatic pendant groups were not so flexible, which made the orientation of polymer chains in-plane direction difficult, resulting in rather random orientation of polymer chains. The dimensional change of PAES-nS membranes was as like as Nafion membranes, which have larger dimensional change in through-plane direction than in-plane direction. This character of the membranes may be propitious for preparation of MEA.| Polymer | IEC (mequiv. g−1) | Water uptake (% W/W) | σ (S cm−1) | Tensile stress (MPa) | Elongation at break (%) | |||
|---|---|---|---|---|---|---|---|---|
| Calculated | Measured | 20 °C | 80 °C | 20 °C | 80 °C | |||
| PAES-2S-a60 | 1.90 | 1.87 | 47.3 | 70.4 | 0.103 | 0.182 | 32.4 | 15.1 |
| PAES-2S-a50 | 1.68 | 1.61 | 35.9 | 42.3 | 0.076 | 0.148 | 37.0 | 12.3 |
| PAES-2S-a40 | 1.43 | 1.40 | 17.0 | 24.4 | 0.029 | 0.069 | 40.0 | 14.9 |
| PAES-2S-b60 | 1.73 | 1.69 | 32.0 | 54.3 | 0.090 | 0.149 | 34.3 | 18.9 |
| PAES-2S-b50 | 1.54 | 1.48 | 29.1 | 36.5 | 0.056 | 0.136 | 39.6 | 16.0 |
| PAES-2S-b40 | 1.33 | 1.29 | 10.5 | 15.4 | 0.026 | 0.048 | 43.3 | 13.9 |
| PAES-3S-40 | 1.93 | 1.89 | 50.0 | 73.0 | 0.110 | 0.192 | 30.9 | 16.5 |
| Nafion 117 | 0.91 | 0.91 | 19.2 | 29.4 | 0.095 | 0.175 | 27 | 327 |
3.5. Proton conductivity
All the PAES-nS membranes displayed high proton conductivity above 10−2 S cm−1 over the temperature range studied (Table 3 and Fig. 9). At low temperature, the proton conductivity of PAES-nS was markedly higher than that of typical aromatic PEMs of which sulfonic acid groups were attached to the backbones.49 The conductivity of PAES-2S-a60 (0.09 S cm−1) and PAES-2S-b60 (0.103 S cm−1) was higher than Nafion 117 at room temperature. The PAES-3S-40 polymer exhibited the highest conductivity at the temperature range studied, due to its highest IEC value. Compared with previous reported results,35 PAES-nS polymers exhibited higher conductivity than SC-SPAE. For example, PAES-2S-b50's (IEC = 1.48 mequiv. g−1) conductivity was 1.48 × 10−1 S cm−1, which was much higher than SC-SPAE80's (IEC = 1.52 mequiv. g−1) conductivity of 3.4 × 10−2 S cm−1. From polymer structure, the electron withdrawing groups (fluorine atom and carbonyl groups) are capable of increasing acidity and serve to high conductivity for the PAES-nS membranes. As shown in Fig. 9, the increase in conductivity was correlated to the increase in IEC values and temperature for PAES-nS. Although owning low IEC value (1.29 mequiv. g−1), the conductivity of PAES-2S-b40 membrane was as high as 2.6 × 10−2 S cm−1 at room temperature. The large side-chain of PAES-nS resulted in its large free volume, which serves to hydrophilic domains, as seen in SAXS data.3.6. Morphology
SAXS was usually carried out to study the internal structure of the ionic polymers including the ionic clusters. As mentioned by Hashimoto and co-workers, these studies indicated the relationship between cluster size and scattering vector (q).50,51 For proton exchange membrane, the phase separation of hydrophilic and hydrophobic domains may lead to the scattering maximum at large angles, which can be assigned to ionic clusters. SAXS profiles of PAES-2S-a50 and PAES-2S-b50 are shown in Fig. 10. The ionic scattering maximum of PAES-2S-a50, PAES-2S-b50 and Nafion 117 was found to have q values of 1.10 nm−1, 1.12 nm−1, and 1.79 nm−1, respectively. The Bragg spacing D (i.e. domain periodicities), referring to the center-to-center distance between two ionic clusters, can be obtained from equation D = 2π/q (where q is the scatter vector).52,53 It can be calculated that the Bragg spacing is of a value of 5.71 nm, 5.60 nm, and 3.50 nm, respectively. Generally, Bragg spacing increases as the content of sulfonic acid groups increase in sulfonated aromatic polymer film.54 PAES-nS polymer film carrying large side chain owned large free volume, and the Bragg spacing was larger than that of Nafion 117. This result then demonstrated that side-chain-style PAES-nS membrane will provide much larger proton transport channel and the transport channel further increased with the increase of side chain length.3.7 Mechanical properties
To evaluate the mechanical properties of PAES-nS polymers, the tensile testing of wet membranes were performed and the results are listed in Table 3. The tensile strength of PAES-nS was observed from 30 to 44 MPa, and the elongation at break was from 12% to 19%. It's assumed that sulfonic acid groups inevitably alter the nature of the polymer backbone, and this made sulfonated polymers exhibit poorer tensile strength as the content of sulfonic acid groups (IEC) increased.4. Conclusions
A novel side chain style multi-sulfonated poly(arylene ether sulfone)s were prepared and they displayed advantageous conductivity and membrane hydrodynamic properties as compared to most of sulfonated aromatic main-chain polymers. Wholly aromatic structure of PAES-nS polymers ensured good thermochemical stability. The unique polymer chemical structure resulted in excellent dimensional stability and high proton conductivity. By intended design, sulfonic acid groups were located around electron withdrawing groups, which was propitious to increase acidity and serve to high conductivity. Additionally, SAXS analyses confirmed ionic domain with the size above 5.5 nm, indicating broad proton transport channels formed.Acknowledgements
The authors would like to thank the China Natural Science Foundation (Grant no. 51103060) and Science and Technology Development Plan of Jilin Province, China (no. 20140309001GX) supports this work.References
- J. Rozière and D. J. Jones, Annu. Rev. Mater. Res., 2003, 33, 503–555 CrossRef.
- O. Savadogo, J. New Mater. Electrochem. Syst., 1998, 1, 047–066 CAS.
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla and J. E. McGrath, Chem. Rev., 2004, 104, 4587–4612 CrossRef CAS.
- M. Rikukawa and K. Sanui, Prog. Polym. Sci., 2000, 25, 1463–1502 CrossRef CAS.
- M. A. Hickner and B. S. Pivovar, Fuel Cells, 2005, 5, 213–229 CrossRef CAS PubMed.
- S. M. J. Zaidi, S. D. Mikhailenko, G. P. Robertson, M. D. Guiver and S. Kaliaguine, J. Membr. Sci., 2000, 173, 17–34 CrossRef CAS.
- S. Kaliaguine, S. D. Mikhailenko, K. P. Wang, P. Xing, G. Robertson and M. Guiver, Catal. Today, 2003, 82, 213–222 CrossRef CAS.
- G. P. Robertson, S. D. Mikhailenko, K. Wang, P. Xing, M. D. Guiver and S. Kaliaguine, J. Membr. Sci., 2003, 219, 113–121 CrossRef CAS.
- P. Xing, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko, K. Wang and S. Kaliaguine, J. Membr. Sci., 2004, 229, 95–106 CrossRef CAS PubMed.
- J. Kerres, A. Ullrich, F. Meier and T. Häring, Solid State Ionics, 1999, 125, 243–249 CrossRef CAS.
- H. Zhang and P. K. Shen, Chem. Rev., 2012, 112, 2780–2832 CrossRef CAS PubMed.
- K. Matsumoto, T. Higashihara and M. Ueda, Macromolecules, 2009, 42, 1161–1166 CrossRef CAS.
- S. Matsumura, A. R. Hlil, C. Lepiller, J. Gaudet, D. Guay and A. S. Hay, Macromolecules, 2008, 41, 277–280 CrossRef CAS.
- S. Matsumura, A. R. Hlil, C. Lepiller, J. Gaudet, D. Guay, Z. Shi, S. Holdcroft and A. S. Hay, Macromolecules, 2008, 41, 281–284 CrossRef CAS.
- K. D. Kreuer, J. Membr. Sci., 2001, 185, 29–39 CrossRef CAS.
- K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637–4678 CrossRef CAS.
- Z. Li, J. Ding, G. P. Robertson and M. D. Guiver, Macromolecules, 2006, 39, 6990–6996 CrossRef CAS.
- H. Ghassemi, G. Ndip and J. E. McGrath, Polymer, 2004, 45, 5855–5862 CrossRef CAS PubMed.
- T. Kobayashi, M. Rikukawa, K. Sanui and N. Ogata, Solid State Ionics, 1998, 106, 219–225 CrossRef CAS.
- N. Asano, K. Miyatake and M. Watanabe, Chem. Mater., 2004, 16, 2841–2843 CrossRef CAS.
- N. Asano, M. Aoki, S. Suzuki, K. Miyatake, H. Uchida and M. Watanabe, J. Am. Chem. Soc., 2006, 128, 1762–1769 CrossRef CAS PubMed.
- Z. Qiu, S. Wu, Z. Li, S. Zhang, W. Xing and C. Liu, Macromolecules, 2006, 39, 6425–6432 CrossRef CAS.
- Y. Chang, G. F. Brunello, J. Fuller, M. L. Disabb-Miller, M. E. Hawley, Y. S. Kim, M. A. Hickner, S. S. Jang and C. Bae, Polym. Chem., 2013, 4, 272–281 RSC.
- T. J. Peckham and S. Holdcroft, Adv. Mater., 2010, 22, 4667–4690 CrossRef CAS PubMed.
- C. Wang, N. Li, D. W. Shin, S. Y. Lee, N. R. Kang, Y. M. Lee and M. D. Guiver, Macromolecules, 2011, 44, 7296–7306 CrossRef CAS.
- R. Nolte, K. Ledjeff, M. Bauer and R. Mülhaupt, J. Membr. Sci., 1993, 83, 211–220 CrossRef CAS.
- Y. Gao, G. P. Robertson, D.-S. Kim, M. D. Guiver, S. D. Mikhailenko, X. Li and S. Kaliaguine, Macromolecules, 2007, 40, 1512–1520 CrossRef CAS.
- P. Xing, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko and S. Kaliaguine, Macromolecules, 2004, 37, 7960–7967 CrossRef CAS.
- Y. Gao, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko, X. Li and S. Kaliaguine, Macromolecules, 2004, 37, 6748–6754 CrossRef CAS.
- H. Liao, G. Xiao and D. Yan, Chem. Commun., 2013, 49, 3979–3981 RSC.
- N. Tan, G. Xiao and D. Yan, Chem. Mater., 2009, 22, 1022–1031 CrossRef.
- X. F. Li, F. P. V. Paoloni, E. A. Weiber, Z.-H. Jiang and P. Jannasch, Macromolecules, 2012, 45, 1447–1459 CrossRef CAS.
- S. Takamuku and P. Jannasch, Adv. Energy Mater., 2012, 2, 129–140 CrossRef CAS PubMed.
- G. Titvinidze, K.-D. Kreuer, M. Schuster, C. C. de Araujo, J. P. Melchior and W. H. Meyer, Adv. Funct. Mater., 2012, 22, 4456–4470 CrossRef CAS PubMed.
- J. Pang, H. Zhang, X. Li and Z. Jiang, Macromolecules, 2007, 40, 9435–9442 CrossRef CAS.
- J. Pang, H. Zhang, X. Li, D. Ren and Z. Jiang, Macromol. Rapid Commun., 2007, 28, 2332–2338 CrossRef CAS PubMed.
- J. Pang, H. Zhang, X. Li, L. Wang, B. Liu and Z. Jiang, J. Membr. Sci., 2008, 318, 271–279 CrossRef CAS PubMed.
- J. Pang, H. Zhang, X. Li, B. Liu and Z. Jiang, J. Power Sources, 2008, 184, 1–8 CrossRef CAS PubMed.
- X. Yue, H. Zhang, W. Chen, Y. Wang, S. Zhang, G. Wang and Z. Jiang, Polymer, 2007, 48, 4715–4722 CrossRef CAS PubMed.
- F. Wang, T. Chen and J. Xu, Macromol. Chem. Phys., 1998, 199, 1421–1426 CrossRef CAS.
- X. Li, H. Na and H. Lu, Chem. Res. Chin. Univ., 2004, 25, 1563–1566 CAS.
- X. Li, H. Na and H. Lu, J. Appl. Polym. Sci., 2004, 94, 1569–1574 CrossRef CAS PubMed.
- S. Feng, K. Shen, Y. Wang, J. Pang and Z. Jiang, J. Power Sources, 2013, 224, 42–49 CrossRef CAS PubMed.
- M. J. Sumner, W. L. Harrison, R. M. Weyers, Y. S. Kim, J. E. McGrath, J. S. Riffle, A. Brink and M. H. Brink, J. Membr. Sci., 2004, 239, 199–211 CrossRef CAS PubMed.
- F. Wang, M. Hickner, Y. S. Kim, T. A. Zawodzinski and J. E. McGrath, J. Membr. Sci., 2002, 197, 231–242 CrossRef CAS.
- Y. S. Kim, M. A. Hickner, L. Dong, B. S. Pivovar and J. E. McGrath, J. Membr. Sci., 2004, 243, 317–326 CrossRef CAS PubMed.
- C. E. Williams, T. P. Russell, R. Jerome and J. Horrion, Macromolecules, 1986, 19, 2877–2884 CrossRef CAS.
- Z. Hu, Y. Yin, S. Chen, O. Yamada, K. Tanaka, H. Kita and K.-I. Okamoto, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2862–2872 CrossRef CAS PubMed.
- K. D. Kreuer, A. Rabenau and W. Weppner, Angew. Chem., Int. Ed. Engl., 1982, 21, 208–209 CrossRef PubMed.
- M. Fujimura, T. Hashimoto and H. Kawai, Macromolecules, 1982, 15, 136–144 CrossRef CAS.
- M. Fujimura, T. Hashimoto and H. Kawai, Macromolecules, 1981, 14, 1309–1315 CrossRef CAS.
- X. Lu, W. P. Steckle and R. A. Weiss, Macromolecules, 1993, 26, 6525–6530 CrossRef CAS.
- B. Heck, P. Arends, M. Ganter, J. Kressler and B. Stühn, Macromolecules, 1997, 30, 4559–4566 CrossRef CAS.
- X. Li, G. Zhang, D. Xu, C. Zhao and H. Na, J. Power Sources, 2007, 165, 701–707 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: GPC profiles of polymer precursors and DSC curves of disulfonated polymers. See DOI: 10.1039/c5ra02107c |
| This journal is © The Royal Society of Chemistry 2015 |