Organic solar cells based on bowl-shaped small-molecules†
Agustín Molina-Ontoriaa,
María Gallegoc,
Luís Echegoyen*a,
Emilio M. Pérezb and
Nazario Martín*bc
aDepartment of Chemistry, University of Texas at El Paso, 79968, El Paso, Texas, USA. E-mail: echegoyen@utep.edu
bIMDEA-nanociencia, C/Faraday 9, Ciudad Universitaria de Cantoblanco, 28049 Madrid, Spain
cDepartamento de Química Orgánica, Universidad Complutense de Madrid, Fac. C.C. Químicas, Av. Complutense s/n, 28040 Madrid, Spain. E-mail: nazmar@ucm.es; Web: http://www.ucm.es/info/fullerene/
First published on 20th March 2015
Abstract
The light absorption ability and morphology of the active components are two of the key factors that determine the energy conversion efficiency in organic solar cells (OSCs). Determining the relative importance of each of these aspects is decisive for the construction of more efficient OSCs. Here we introduce two π-extended derivatives of tetrathiafulvalene as electron donors for solution-processed small-molecule bulk-heterojunction solar cells. Both of them exhibit similar bowl-shaped geometry, excellent electron-donor characteristics and relatively large association constants with fullerenes in solution (on the order of 104 M−1 for truxTTF and 103 M−1 for truxTTF-CO in several solvents at room temperature). The substitution of one dithiole ring in truxTTF-CO for a carbonyl results in an intramolecular push–pull effect, which enhances its light-harvesting properties, with the onset of absorbance reaching 650 nm. The introduction of a third dithiole ring, results in a more pronounced concave shape in truxTTF, allowing a better self-assembly with fullerenes which in turn leads to a more favourable morphology. However, the light-absorption ability of truxTTF is limited to ca. 500 nm. We prepared bulk-heterojunction solar cells using phenyl-C60-butyric acid methyl ester (PC61BM) and PC71BM as electron-acceptors and bowl-shaped truxTTF and truxTTF-CO as electron-donors. The devices prepared utilizing truxTTF performed significantly better (PCE up to 1.77% with PC71BM and 0.92% with PC61BM) than the truxTTF-CO counterpart (PCE up to 1.19% with PC71BM and 0.56% with PC61BM).
Introduction
The photoactive layer of a bulk-heterojunction solar cell (BHJ) is composed of a blend of either a π-conjugated semiconductor polymer1,2 or small-molecule3,4 as electron donor and a fullerene derivative or another acceptor molecule, such as diketopyrrolopyrrole (DPP), perylenediimide (PDI) or fluoranthene-fused imide (FFI), as electron acceptor. An interpenetrating network at the nanometer scale (shorter than the exciton diffusion length ≈3–10 nm) of these two components is crucial for efficient exciton diffusion to the interfaces, followed by exciton dissociation into charges. Formation of percolating pathways for electrons and holes to be efficiently collected at the external electrodes is also needed to avoid undesired charge recombination processes. Therefore, control of the morphology of the active layer is a key issue in the preparation of BHJ solar cells, given its critical influence on the device performance.5–11 There are several macroscopic methods to assist the control of the morphology, including the use of solvent additives12–14 or solvent/thermal annealing.15–22Moreover, a molecular approach has been used to control the phase segregation, for instance, by self-assembling fullerene derivatives,23,24 by using the side chains of the polymers to accommodate the acceptor moieties25–27 or by means of hydrogen-bonding.28–31 However, in these macroscopic and molecular approaches, the donor and acceptor moieties act individually, since there are no specific interactions between them. On the other hand, a supramolecular approach in which both components interact through their complementary shapes has been reported only recently.32–34 Coronene derivatives (donor) were found to form supramolecular complexes with PC71BM (acceptor) through dispersion-type concave–convex interactions. Both materials were used in order to control the morphology of thin-film solar cells by self-assembly, yielding devices with power conversion efficiency (PCE) values of up to 2.6%.
In 2007, truxene-tetrathiafulvalene (truxTTF) emerged as an excellent supramolecular partner for recognition of C60 as well as C70.35–38 TruxTTF features a truxene core, which is decorated with three covalently linked dithiole units (Fig. 1). The introduction of these dithiole rings in an all-cis configuration leads to a deviation from planarity of the truxene core, which adopts a bowl-shape geometry (Fig. 2a).37 Its relatively large concave aromatic surface allows for the self-assembly with fullerenes and fullerene derivatives, such as PC61BM and PC71BM, with binding constants (Ka) in the range of 104 M−1 (Fig. S1, ESI†). Recently, a new truxTTF-like derivative truxTTF-CO was synthetized.38 TruxTTF-CO shows enhanced absorption properties in thin film, compared to truxTTF, while retaining the ability to bind with C60, albeit with a significantly lower binding constant (Ka ≈ 103 M−1) with PC61BM and PC71BM (Fig. S2, ESI†). TruxTTF-CO exhibits a push–pull structure where one dithiole ring is substituted for a weak electron-withdrawing carbonyl group. As a consequence, a new bathocromically shifted intramolecular charge-transfer band is observed in its electronic absorption spectrum, which expands to ca. 650 nm.
 | ||
| Fig. 1 Chemical structures of bowl-shape electron donors truxTTF and truxTTF-CO and electron acceptors phenyl-C60-butyric acid methyl ester (PC61BM) and phenyl-C70-butyric acid methyl ester (PC71BM). | ||
These characteristics make truxTTF and truxTTF-CO ideal candidates to investigate the relative importance of concave–convex interactions on the morphology and efficiency of OSCs. When using an electron acceptor with limited absorption properties, such as PC61BM, morphology is a very important parameter to control. The stronger interactions (π–π, charge transfer and van der Waals interactions) between the concave face of the truxTTF and the convex face of the PC61BM could lead to better self-organization of the photoactive layer (Fig. 2). These interactions between the donor and the acceptor are even stronger when using larger fullerene cages, such as PC71BM, which could promote a more successful formation of the supramolecular complex and consequently well-defined donor/acceptor junctions.
 | ||
| Fig. 2 (a) X-ray crystal structure of truxTTF37 and (b) X-ray crystal structure of truxTTF-CO.38 Carbon atoms are shown in green, sulfur in yellow and oxygen in red. | ||
Herein, we demonstrate that, at least for our particular systems, the molecular order induced by the concave–convex supramolecular interactions has a strong impact on the morphology and PCE values.
Results and discussion
Fig. 3 shows optical absorption spectra of truxTTF and truxTTF-CO in dilute CHCl3 solution and spin-coated thin films. In solution, truxTTF-CO absorbs over a wider spectral range (300–655 nm) than truxTTF. TruxTTF-CO exhibits maximum absorption band (λmax) at 400 nm and an intramolecular charge transfer (CT) band with low absorption at 550 nm, which is originated from electronic transitions from the dithiole rings to the carbonyl group.37 On the other hand, truxTTF displays a sharper absorption peak centered at 449 nm, reflecting the lack of an intramolecular push–pull effect and higher loss of planarity. Moreover, both molecules exhibit similar absorption coefficient (ε) values (Fig. S3, ESI†). In the solid state, truxTTF and truxTTF-CO show broader absorptions, compared with those in solution, extending the absorption to 618 and 677 nm, for truxTTF and truxTTF-CO, respectively. The optical band gaps are estimated to be 2.00 eV and 1.83 eV for truxTTF and truxTTF-CO respectively. Although not as pronounced as in solution, truxTTF-CO still absorbs over a wider spectral range in the thin films. | ||
| Fig. 3 UV-vis absorption spectra of truxTTF and truxTTF-CO in diluted chloroform solution and in thin films. | ||
The electrochemical properties of truxTTF and truxTTF-CO were analyzed by cyclic voltammetry (CV).37,38 The highest occupied molecular orbital (HOMO) for truxTTF and truxTTF-CO were estimated from the oxidation onset potential to be −4.95 eV and −5.07 eV. The lowest unoccupied molecular orbital (LUMO) of truxTTF-CO was calculated from the onset reduction potential to be −3.4 eV. TruxTTF exhibits a LUMO of −2.95 eV, which was estimated from the optical band gap. The deeper HOMO level of truxTTF-CO should lead to an enhancement in the Voc (Fig. 4b).
 | ||
| Fig. 4 (a) Device structure of the conventional sandwich solar cells (ITO/PEDOT:PSS/active layer/Al) and (b) energy-level diagram of the device. | ||
To explore the properties of truxTTF and truxTTF-CO in photovoltaic devices, solar cells were fabricated using the conventional sandwich structure of ITO/PEDOT:PSS/donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acceptor/Al or Ca/Al by using a varying weight ratio of the donor/PC61BM from 1
acceptor/Al or Ca/Al by using a varying weight ratio of the donor/PC61BM from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 in a chlorobenzene
6 in a chlorobenzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ortho-dichlorobenzene (o-DCB) (1
ortho-dichlorobenzene (o-DCB) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixture. These experiments were performed under ambient atmosphere employing AM 1.5 G simulated illumination at an intensity of 100 mW cm−2. The current density–voltage (J–V) characteristics and the external quantum efficiency (EQE) for the conventional device architecture ITO/PEDOT/active layer/Al are shown in Fig. 5a–d and the performance parameters are summarized in Table 1 as a function of the weight ratios of donor
3) mixture. These experiments were performed under ambient atmosphere employing AM 1.5 G simulated illumination at an intensity of 100 mW cm−2. The current density–voltage (J–V) characteristics and the external quantum efficiency (EQE) for the conventional device architecture ITO/PEDOT/active layer/Al are shown in Fig. 5a–d and the performance parameters are summarized in Table 1 as a function of the weight ratios of donor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM.
PC61BM.
| Active layer | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE [highest] (%) |
|---|---|---|---|---|
truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
0.53 ± 0.03 | 4.34 ± 0.21 | 30.1 ± 0.21 | 0.69 ± 0.1 [0.72] |
truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.57 ± 0.01 | 4.42 ± 0.17 | 30.2 ± 0.19 | 0.76 ± 0.1 [0.80] |
truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.63 ± 0.02 | 4.28 ± 0.10 | 31.2 ± 0.11 | 0.81 ± 0.1 [0.92] |
truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
0.40 ± 0.01 | 3.13 ± 0.11 | 28.1 ± 0.18 | 0.34 ± 0.04 [0.36] |
truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.50 ± 0.02 | 3.24 ± 0.21 | 28.7 ± 0.26 | 0.47 ± 0.1 [0.56] |
truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.50 ± 0.02 | 3.31 ± 0.23 | 27.7 ± 0.18 | 0.44 ± 0.04 [0.47] |
It is important to note that the D/A ratio strongly affects the Voc, which can be attributed to a more effective supramolecular complexation (lower association constants for truxTTF-CO compared to truxTTF were obtained using PC61BM), but only slightly affects the fill factor (FF) and the short-circuit current density (Jsc). A photovoltaic device using truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) w/w ratio yielded an average power conversion efficiency of 0.81%, with a Voc of 0.63 V, a short-circuit current density (Jsc) of 4.28 mA cm−2 and a FF of 31.2%. Average values were taken from 10 devices. In addition, the overall efficiency and the Voc decreased upon further increasing the PC61BM ratio, but the FF and the Jsc remained almost constant. On the other hand, the devices with a blend of truxTTF-CO
2) w/w ratio yielded an average power conversion efficiency of 0.81%, with a Voc of 0.63 V, a short-circuit current density (Jsc) of 4.28 mA cm−2 and a FF of 31.2%. Average values were taken from 10 devices. In addition, the overall efficiency and the Voc decreased upon further increasing the PC61BM ratio, but the FF and the Jsc remained almost constant. On the other hand, the devices with a blend of truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) w/w ratio exhibited a PCE of 0.56%, with Jsc of 3.5 mA cm−2 and with significantly lower FF values than those observed for truxTTF, which is indicative of worse capacity to dissociate excitons and extract charge carriers and an unfavorable morphology. The bowl shaped contour of these donors favor a strong overlap with the acceptor, but such overlap also enables rapid charge recombination.
4) w/w ratio exhibited a PCE of 0.56%, with Jsc of 3.5 mA cm−2 and with significantly lower FF values than those observed for truxTTF, which is indicative of worse capacity to dissociate excitons and extract charge carriers and an unfavorable morphology. The bowl shaped contour of these donors favor a strong overlap with the acceptor, but such overlap also enables rapid charge recombination.
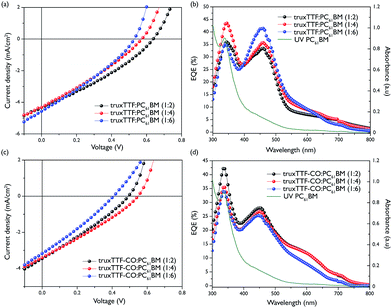
The external quantum efficiency (EQE) curves for devices based on different donor/acceptor (D/A) ratios, displayed in Fig. 5b, were investigated under monochromatic light. Both donor systems when blended with PC61BM exhibited a narrow spectral response, ranging from 300 to 700 nm. The devices containing truxTTF and PC61BM exhibited EQEs of 43% and 36% at 343 and 459 nm, respectively. The former corresponds to the photocurrent arising from the PC61BM and the latter corresponds to the contribution coming from the truxTTF. On the other hand, devices incorporating a blend of truxTTF-CO/PC61BM exhibited a broader but lower intensity photo-response with a shoulder at 594 nm, due to the broader light-harvesting abilities of the truxTTF-CO. The Jsc obtained from the J–V measurements and the integrated current densities from the EQEs are in good agreement, within ±5%.
To further explore the photovoltaic performances of truxTTF and truxTTF-CO donor compounds, solar cells were investigated with a different electron acceptor, namely PC71BM (Fig. 6). It is known that PC61BM absorbs over a narrower range and has lower extinction coefficient than PC71BM in the visible region. In thin film, PC71BM displays a broad absorption from 300 to 750 nm, whereas PC61BM has limited photo-response in the same region.
Photovoltaic devices incorporating truxTTF exhibited a higher power conversion efficiency for a blend ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 w/w, with an Voc of 0.6 V, a short-circuit current (Jsc) of 8.11 mA cm−2 and a FF of 33.1%, for an overall PCE average of 1.61% and a maximum PCE of 1.77%, which corresponds to an improvement of almost 100% with respect to those devices containing PC61BM as the electron acceptor (Table 2).
6 w/w, with an Voc of 0.6 V, a short-circuit current (Jsc) of 8.11 mA cm−2 and a FF of 33.1%, for an overall PCE average of 1.61% and a maximum PCE of 1.77%, which corresponds to an improvement of almost 100% with respect to those devices containing PC61BM as the electron acceptor (Table 2).
| Active layer | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE [highest] (%) |
|---|---|---|---|---|
truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
0.55 ± 0.01 | 4.81 ± 0.37 | 29.8 ± 0.17 | 0.80 ± 0.1 [0.89] |
truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
0.60 ± 0.03 | 8.11 ± 0.22 | 33.1 ± 0.28 | 1.61 ± 0.1 [1.77] |
truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.53 ± 0.01 | 6.38 ± 0.12 | 31.9 ± 0.24 | 1.07 ± 0.1 [1.15] |
truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.58 ± 0.02 | 4.87 ± 0.10 | 30.8 ± 0.11 | 0.83 ± 0.1 [0.88] |
truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
0.51 ± 0.01 | 1.49 ± 0.14 | 26.3 ± 0.15 | 0.2 ± 0.02 [0.22] |
truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
0.61 ± 0.02 | 5.78 ± 0.31 | 30.2 ± 0.22 | 1.06 ± 0.1 [1.19] |
truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.56 ± 0.02 | 3.82 ± 0.25 | 29.0 ± 0.21 | 0.62 ± 0.04 [0.68] |
When the D/A ratio was adjusted from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 to 1
6 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the Jsc decreased from 8.11 to 4.87 mA cm−2 and the FF decreases from 33.1 to 30.8%. This experimental finding could be explained in terms of aggregation of truxTTF molecules when the amount of the donor increases, which results in the formation of larger domains of truxTTF, and therefore unsuccessful creation of the supramolecular complex.33 In contrast, the best photovoltaic performance for truxTTF-CO was provided by the blend with a 1
2, the Jsc decreased from 8.11 to 4.87 mA cm−2 and the FF decreases from 33.1 to 30.8%. This experimental finding could be explained in terms of aggregation of truxTTF molecules when the amount of the donor increases, which results in the formation of larger domains of truxTTF, and therefore unsuccessful creation of the supramolecular complex.33 In contrast, the best photovoltaic performance for truxTTF-CO was provided by the blend with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w ratio (average PCE 1.06%, maximum of 1.19%), with a Voc of 0.61 V, Jsc of 5.78 mA cm−2 and a FF of 30.2%. The Voc values obtained are consistent with the difference between the donor HOMO and the LUMO of the PC71BM in both cases. Furthermore, the photovoltaic properties were investigated employing the best ratios for each donor molecule, with the device architecture of ITO/PEDOT:PSS/active layer/Ca/Al. The introduction of Ca as an electron transport layer (ETL) provides a higher built-in potential and a more efficient electron transport and collection process (increasing the FF). As expected, a remarkable improvement of 66 mV in the Voc and more than 3% in the FF was achieved in a blend of truxTTF
4 w/w ratio (average PCE 1.06%, maximum of 1.19%), with a Voc of 0.61 V, Jsc of 5.78 mA cm−2 and a FF of 30.2%. The Voc values obtained are consistent with the difference between the donor HOMO and the LUMO of the PC71BM in both cases. Furthermore, the photovoltaic properties were investigated employing the best ratios for each donor molecule, with the device architecture of ITO/PEDOT:PSS/active layer/Ca/Al. The introduction of Ca as an electron transport layer (ETL) provides a higher built-in potential and a more efficient electron transport and collection process (increasing the FF). As expected, a remarkable improvement of 66 mV in the Voc and more than 3% in the FF was achieved in a blend of truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM, for a maximum efficiency of 1.77%. Surprisingly, no improvement was observed for the blend containing truxTTF-CO and PC71BM (1
PC71BM, for a maximum efficiency of 1.77%. Surprisingly, no improvement was observed for the blend containing truxTTF-CO and PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w).
4 w/w).
Furthermore, in order to get further insights into the supramolecular assembly in thin film, 1,8-diiodooctane (DIO) was added. It was reported that the addition of DIO can hinder or inhibit the interactions between complementary faces of the donor and the acceptor.33 As expected, the PCE for truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) w/w and truxTTF-CO
6) w/w and truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) w/w decreased when the DIO was present (Fig. S4 and S5, ESI†).
4) w/w decreased when the DIO was present (Fig. S4 and S5, ESI†).
It is important to emphasize that the FF was higher when truxTTF was used as electron donor. The FF is known to depend heavily on morphology/charge-recombination39–42 and, in our case, truxTTF and PC71BM blends lead to higher efficiencies when compared to truxTTF-CO.
Fig. 6b and d show the external quantum efficiency (EQE) curves for those devices based on varying D/A ratios. Both spectra of donor units blended with PC71BM exhibited a broader spectral response than those with PC61BM, ranging from 300 to 750 nm, whereas the mixture of truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM at 1
PC71BM at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 w/w ratio reached the highest value of EQE (55%) at 463 nm. When the donor ratio decreases to 1
6 w/w ratio reached the highest value of EQE (55%) at 463 nm. When the donor ratio decreases to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 or 1
4 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the contribution to the EQE is mainly from PC71BM, but when the ratio is increased to 1
6, the contribution to the EQE is mainly from PC71BM, but when the ratio is increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 the contribution of the donor is similar to the contribution of the PC71BM. For the blend based on truxTTF
2 the contribution of the donor is similar to the contribution of the PC71BM. For the blend based on truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 w/w), the higher experimental Jsc values were 8.31 mA cm−2 whereas the estimated value of Jsc from the EQE measurements was 8.29 mA cm−2. The corresponding devices containing truxTTF-CO
6 w/w), the higher experimental Jsc values were 8.31 mA cm−2 whereas the estimated value of Jsc from the EQE measurements was 8.29 mA cm−2. The corresponding devices containing truxTTF-CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM displayed a lower EQE (41% at 460 nm for 1
PC71BM displayed a lower EQE (41% at 460 nm for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio).
4 ratio).
The active layer morphologies incorporating truxTTF and truxTTF-CO, were characterized by atomic force microscopy (AFM) in tapping mode, using the best composition for each device, as shown in Fig. 7. The images reveal that truxTTF presents a more favourable morphology when compared to that of truxTTF-CO.
The surface topographies of truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1
PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) and truxTTF
6) and truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) blend films exhibit rather uniform nanometer-sized features with a root-mean-square roughness (rms) value of 0.33 nm and 0.31 nm, respectively. On the other hand, blends containing truxTTF-CO with PC61BM and PC71BM display coarser topographies, with higher rms values of 0.47 nm and 0.43 nm, respectively. In addition, the truxTTF
6) blend films exhibit rather uniform nanometer-sized features with a root-mean-square roughness (rms) value of 0.33 nm and 0.31 nm, respectively. On the other hand, blends containing truxTTF-CO with PC61BM and PC71BM display coarser topographies, with higher rms values of 0.47 nm and 0.43 nm, respectively. In addition, the truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM reveals the formation of slightly larger and better defined morphological features (10–50 nm), measured from cross-sectional profiles (Fig. S6, ESI†), which is beneficial for exciton dissociation and charge transport.43,44
PC71BM reveals the formation of slightly larger and better defined morphological features (10–50 nm), measured from cross-sectional profiles (Fig. S6, ESI†), which is beneficial for exciton dissociation and charge transport.43,44
These experimental findings could be accounted for by the better miscibility of truxTTF with PC71BM which eventually results in an enhance device performance.
Conclusions
In conclusion, a less-explored supramolecular approach has been used for the preparation of small-molecule solar cells. The highest PCE based on a truxTTF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) w/w ratio was 1.77%, while incorporating truxTTF-CO yielded 1.19%. Although, both of them possess similar bowl-shape geometries that allow the formation of supramolecular complexes when blended with PC61BM or PC71BM, they show different binding constants with fullerenes and also different optical properties. Despite the broader absorption and the deeper HOMO of truxTTF-CO, the stronger non-covalent interactions between the concave shape of the electron donating truxTTF and the convex surface of the fullerene derivatives lead to higher Jsc and FF, probably as a result of the more favorable morphology of the blends.
6) w/w ratio was 1.77%, while incorporating truxTTF-CO yielded 1.19%. Although, both of them possess similar bowl-shape geometries that allow the formation of supramolecular complexes when blended with PC61BM or PC71BM, they show different binding constants with fullerenes and also different optical properties. Despite the broader absorption and the deeper HOMO of truxTTF-CO, the stronger non-covalent interactions between the concave shape of the electron donating truxTTF and the convex surface of the fullerene derivatives lead to higher Jsc and FF, probably as a result of the more favorable morphology of the blends.
Acknowledgements
L.E. thanks the Robert A. Welch Foundation for an endowed chair, grant #AH-0033, the US National Science Foundation, grant DMR-1205302 (PREM Program) and the Air Force Office of Scientific Research (grants FA9550-12-1-0053 and FA9550-12-1-0468) for the generous financial support. We are thankful to A. Soubrié (Centro de Microscopía y Citometría, UCM) for AFM imaging. M.G. acknowledges the Spanish Ministry of Education, Culture and Sport (MECD) for a FPU studentship. Financial support by the Ministerio de Economia y Competitividad (MINECO) of Spain (project CTQ2011-24652 and CTQ2011-25714), the CAM (FOTOCARBON project S2013/MIT-2841), PRI-PRIBUS (2011-1067) and the European Research Council (ERC-320441-Chirallcarbon) is acknowledged. N.M. thanks to Alexander von Humboldt Foundation.Notes and references
- C. J. Brabec, S. Gowrisanker, J. J. M. Halls, D. Laird, S. Jia and S. P. Williams, Adv. Mater., 2010, 22, 3839–3856 CrossRef CAS PubMed.
- J.-T. Chen and C.-S. Hsu, Polym. Chem., 2011, 2, 2707–2722 RSC.
- A. Mishra and P. Bauerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245–4272 RSC.
- B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- A. J. Moulé and K. Meerholz, Adv. Funct. Mater., 2009, 19, 3028–3036 CrossRef.
- G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323–1338 CrossRef CAS.
- F. Yang, M. Shtein and S. R. Forrest, Nat. Mater., 2004, 4, 37–41 CrossRef.
- J. Peet, M. L. Senatore, A. J. Heeger and G. C. Bazan, Adv. Mater., 2009, 21, 1521–1527 CrossRef CAS.
- J. L. Delgado, P.-A. Bouit, S. Filippone, M. A. Herranz and N. Martín, Chem. Commun., 2010, 46, 4853–4865 RSC.
- Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Chem. Rev., 2014, 114, 7006–7043 CrossRef CAS PubMed.
- J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497–500 CrossRef CAS PubMed.
- S. J. Lou, J. M. Szarko, T. Xu, L. Yu, T. J. Marks and L. X. Chen, J. Am. Chem. Soc., 2011, 133, 20661–20663 CrossRef CAS PubMed.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619–3623 CrossRef CAS PubMed.
- G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864–868 CrossRef CAS.
- G. Li, Y. Yao, H. Yang, V. Shrotriya, G. Yang and Y. Yang, Adv. Funct. Mater., 2007, 17, 1636–1644 CrossRef CAS.
- W. Ma, C. Yang, X. Gong, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2005, 15, 1617–1622 CrossRef CAS.
- M. Campoy-Quiles, T. Ferenczi, T. Agostinelli, P. G. Etchegoin, Y. Kim, T. D. Anthopoulos, P. N. Stavrinou, D. D. C. Bradley and J. Nelson, Nat. Mater., 2008, 7, 158–164 CrossRef CAS PubMed.
- S. Miller, G. Fanchini, Y.-Y. Lin, C. Li, C.-W. Chen, W.-F. Su and M. Chhowalla, J. Mater. Chem., 2008, 18, 306–312 RSC.
- G. Wei, S. Wang, K. Sun, M. E. Thompson and S. R. Forrest, Adv. Energy Mater., 2011, 1, 184–187 CrossRef CAS.
- G. Wei, R. R. Lunt, K. Sun, S. Wang, M. E. Thompson and S. R. Forrest, Nano Lett., 2010, 10, 3555–3559 CrossRef CAS PubMed.
- T. A. Bull, L. S. C. Pingree, S. A. Jenekhe, D. S. Ginger and C. K. Luscombe, ACS Nano, 2009, 3, 627–636 CrossRef CAS PubMed.
- R. D. Kennedy, A. L. Ayzner, D. D. Wanger, C. T. Day, M. Halim, S. I. Khan, S. H. Tolbert, B. J. Schwartz and Y. Rubin, J. Am. Chem. Soc., 2008, 130, 17290–17292 CrossRef CAS PubMed.
- C. J. Tassone, A. L. Ayzner, R. D. Kennedy, M. Halim, M. So, Y. Rubin, S. H. Tolbert and B. J. Schwartz, J. Phys. Chem. C, 2011, 115, 22563–22571 CAS.
- N. C. Cates, R. Gysel, Z. Beiley, C. E. Miller, M. F. Toney, M. Heeney, I. McCulloch and M. D. McGehee, Nano Lett., 2009, 9, 4153–4157 CrossRef CAS PubMed.
- C. Bruner, N. C. Miller, M. D. McGehee and R. H. Dauskardt, Adv. Funct. Mater., 2013, 23, 2863–2871 CrossRef CAS.
- N. C. Miller, S. Sweetnam, E. T. Hoke, R. Gysel, C. E. Miller, J. A. Bartelt, X. Xie, M. F. Toney and M. D. McGehee, Nano Lett., 2012, 12, 1566–1570 CrossRef CAS PubMed.
- K. H. Lam, T. R. B. Foong, Z. E. Ooi, J. Zhang, A. C. Grimsdale and Y. M. Lam, ACS Appl. Mater. Interfaces, 2013, 5, 13265–13274 CAS.
- B. M. Schulze, N. T. Shewmon, J. Zhang, D. L. Watkins, J. P. Mudrick, W. Cao, R. Bou Zerdan, A. J. Quartararo, I. Ghiviriga, J. Xue and R. K. Castellano, J. Mater. Chem. A, 2014, 2, 1541–1549 CAS.
- A. Ruiz-Carretero, T. Aytun, C. J. Bruns, C. J. Newcomb, W.-W. Tsai and S. I. Stupp, J. Mater. Chem. A, 2013, 1, 11674–11681 CAS.
- C.-H. Huang, N. D. McClenaghan, A. Kuhn, J. W. Hofstraat and D. M. Bassani, Org. Lett., 2005, 7, 3409–3412 CrossRef CAS PubMed.
- S. J. Kang, J. B. Kim, C.-Y. Chiu, S. Ahn, T. Schiros, S. S. Lee, K. G. Yager, M. F. Toney, Y.-L. Loo and C. Nuckolls, Angew. Chem., Int. Ed., 2012, 51, 8594–8597 CrossRef CAS PubMed.
- S. J. Kang, S. Ahn, J. B. Kim, C. Schenck, A. M. Hiszpanski, S. Oh, T. Schiros, Y.-L. Loo and C. Nuckolls, J. Am. Chem. Soc., 2013, 135, 2207–2212 CrossRef CAS PubMed.
- S. Xiao, S. J. Kang, Y. Wu, S. Ahn, J. B. Kim, Y.-L. Loo, T. Siegrist, M. L. Steigerwald, H. Li and C. Nuckolls, Chem. Sci., 2013, 4, 2018–2023 RSC.
- E. M. Pérez and N. Martín, Chem. Soc. Rev., 2008, 37, 1512–1519 RSC.
- M. Gallego, J. Calbo, J. Aragó, R. M. K. Calderon, F. H. Liquido, A. Iwamoto, A. K. Greene, E. A. Jackson, E. M. Pérez, E. Ortí, D. M. Guldi, L. T. Scott and N. Martín, Angew. Chem.,Int. Ed., 2014, 53, 2170–2175 CrossRef CAS PubMed.
- E. M. Pérez, M. Sierra, L. Sánchez, M. R. Torres, R. Viruela, P. M. Viruela, E. Orti and N. Martín, Angew. Chem., Int. Ed., 2007, 46, 1847–1851 CrossRef PubMed.
- H. Isla, B. Grimm, E. M. Pérez, M. Rosario Torres, M. A. Herranz, R. Viruela, J. Aragó, E. Orti, D. M Guldi and N. Martín, Chem. Sci., 2012, 3, 498–508 RSC.
- V. D. Mihailetchi, H. X. Xie, B. de Boer, L. J. A. Koster and P. W. M. Blom, Adv. Funct. Mater., 2006, 16, 699–708 CrossRef CAS.
- B. Qi and J. Wang, Phys. Chem. Chem. Phys., 2013, 15, 8972–8982 RSC.
- D. Gupta, S. Mukhopadhyay and K. S. Narayan, Sol. Energy Mater. Sol. Cells, 2010, 94, 1309–1313 CrossRef CAS PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- C. R. McNeill, S. Westenhoff, C. Groves, R. H. Friend and N. C. Greenham, J. Phys. Chem. C, 2007, 111, 19153–19160 CAS.
- Y. Yao, J. Hou, Z. Xu, G. Li and Y. Yang, Adv. Funct. Mater., 2008, 18, 1783–1789 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02073e |
| This journal is © The Royal Society of Chemistry 2015 |