Effect of organic solvent and Brønsted acid on 5-hydroxymethylfurfural preparation from glucose over CrCl3†
Yan Yang,
Wentao Liu,
Ningning Wang,
Haijun Wang*,
Zhanxin Song and
Wei Li
The Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, Jiangsu, China. E-mail: wanghj329@outlook.com; Tel: +86 13382888162
First published on 6th March 2015
Abstract
In the present work, the influence of organic solvent on the mechanism of 5-hydroxymethylfurfural (HMF) preparation from glucose over CrCl3 and the role of Brønsted acid (N-methyl-2-pyrrolidone hydrogen sulfate ([NMP]HSO4), N-methyl-2-pyrrolidone bromide ([NMP]Br), N-methyl-2-pyrrolidone chlorine ([NMP]Cl), H2SO4, HBr and HCl) during the reaction were researched by a complementary computational and experimental study. It was found that dimethyl sulfoxide (DMSO) gave the lowest conversion of glucose by surrounding CrCl3, forming a six-coordinated structure (CrCl3–3DMSO). Glucose conversion in N,N-dimethylformamide (DMF) was not selective. N,N-Dimethylacetamide (DMA) and n-butyl alcohol exhibited superior selectivity towards HMF from glucose. Then the role of different Brønsted acids in DMA was expounded. On increasing the dosage of [NMP]HSO4, glucose conversion decreased. A computational study found that HSO4− could also combine with CrCl3, forming six-coordinate complexes. Addition of [NMP]Br and [NMP]Cl accelerated the generation of HMF significantly but didn’t increase the yield. An experimental method preliminarily confirmed that they were mainly responsible for fructose dehydration to HMF. A subsequent computational study further verified that the two kinds of ILs had no effect on glucose isomerization.
1. Introduction
With the rapid development of the global economy and increasing demands for energy, the utilization of biomass which is abundant, renewable, CO2 neutral and can be treated as a feedstock for the production of platform chemicals1 has attracted wide attention. It is important to seek bio-based chemicals from carbohydrate chemistry and the mineral oil-based industrial chemistry. Furan derivatives, such as 5-hydroxymethylfurfural (HMF), are known as important intermediates for preparing fine chemicals, pharmaceuticals, plastic resins, liquid transportation fuels, etc.2 Recently, cellulosic biomass conversion to platform chemicals has gained significant attention. Fructose,3–6 glucose7,8 and cellulose9–11 have been used for the preparation of HMF successively. High yields of HMF could be easily obtained from dehydration of fructose in ionic liquid,12 and using organic solvents especially dimethyl sulfoxide (DMSO).13 However, sucrose, starch and cellulose are more widely available raw materials for HMF production. Among these materials cellulose is highly attractive because of its low price and inedibility.14,15 All the polysaccharides mentioned above contain a glucose monomer, thus the development of a simple and effective process to transform glucose into HMF would be of great significance. Up to now, organic solvents,16 ionic liquids (IL)17,18 and even water19 have been used as reaction medium. In addition, various catalysts have been developed for the production of HMF from the dehydration of glucose, including Lewis acid catalysts,20 basic ionic liquids (ILs),21 zeolites,22 chromium(0) nanoparticles,7 Sn-Mont catalysts,23 phosphate-immobilized anatase TiO2,24 and so on. Among all the catalytic systems studied, organic solvent–CrCl3 or CrCl2, IL–Lewis acid systems are the most efficient. Zhang and co-workers demonstrated a significant HMF yield of 70% from glucose by use of CrCl2 in 1-ethyl-3-methylimidazolium chloride ([EMIM]Cl) ionic liquid (IL).25 Han et al.20 reported that SnCl4 in 1-ethyl-3-methylimidazolium tetrafluoroborate ([EMIM]BF4) enabled the synthesis of HMF from glucose in a good yield of 61%. All of the systems seem to be closely dependent on organic solvent, IL and metal halide. Each of these has different effects on HMF preparation. For example, DMSO can stabilize HMF,26 a metal chloride especially CrCl3 or CrCl2 can promote glucose isomerization to fructose.25 It is necessary to have an insight into the structural and coordination properties of the catalytically relevant species, as well as their complexation with carbohydrates at a molecular level to design novel and green catalytic systems for chemical transformations of lignocellulosic biomass. Qian investigated the mechanisms and energetics for the acid catalyzed β-D-glucose conversion to HMF in water.27 Hensen and co-workers have specialized in investigating the structural and coordination properties of copper(II) and chromium(II) chlorides, their complexes with glucose in dialkylimidazolium chloride ([RMIM]Cl) ionic liquids and the mechanism of glucose dehydration in a MeCl2–[EMIM]Cl (Me = Cr, Cu and Fe) system by a combination of kinetic experiments and density functional theory (DFT) calculations.28–31 They demonstrated that [MeCl4]2− complexes were the reactive species in the MeCl2-catalyzed glucose dehydration in dialkylimidazolium chloride ionic liquid. The catalytic activity of MeCl3 (Me = W, Mo and Fe) in 1-butyl-3-methylimidazolium chloride ([BMIM]Cl) was also compared using DFT calculations.32However, the effect of organic solvent on glucose conversion and whether acidic ILs affect glucose isomerization have been rarely studied. So in our work, N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), N,N-dimethylacetamide (DMA) and n-butyl alcohol were chosen as reaction medium, and CrCl3 served as catalyst. N-Methyl-2-pyrrolidone hydrogen sulfate ([NMP]HSO4), N-methyl-2-pyrrolidone bromide ([NMP]Br), N-methyl-2-pyrrolidone chlorine ([NMP]Cl), H2SO4, HBr, and HCl were treated as cocatalysts. The advantages of the ILs used in this study are easy preparation and low dosage. Experimental and computational approaches were combined to unravel the mechanism of action of organic solvent and Brønsted acid.
2. Experimental section
2.1 Materials
D-Glucose (Glu, AR), CrCl3 (AR), N,N-dimethylformamide (DMF, AR), N,N-dimethylacetamide (DMA, AR), dimethyl sulfoxide (DMSO, AR), n-butyl alcohol (AR), N-methylpyrrolidone and other chemicals (NMP, AR) were all commercial products from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Sulfuric acid (98%), hydrochloric acid (38%), hydrobromic acid (40%) and other chemicals (AR) are commercially available and used without further purification unless otherwise stated.2.2 Synthesis of cocatalysts
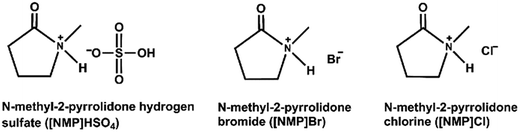
[NMP]HSO4 was prepared by dropping concentrated sulfuric acid (0.12 mol) slowly into N-methyl-2-pyrrolidone (0.1 mol) at 0–5 °C and stirring for 4 h at room temperature. The unreacted chemical reagents and other impurities were removed by vacuum distillation, and the obtained product [NMP]HSO4 was washed with ethyl acetate (3 × 10 mL) and dried at 60 °C under vacuum for 2 days.33–35 1H NMR (400 MHz, D2O): δH (ppm) = 2.01–2.09 (m, 2H), 2.42–2.48 (t, 2H), 2.84 (s, 3H), 3.50–3.54 (t, 2H). The synthetic processes for [NMP]Cl, [NMP]Br were similar to [NMP]HSO4. The structures of the ILs prepared are shown in Fig. 1.2.3 UV-vis acidity evaluation
The Brønsted acidity was evaluated from the determination of the Hammett acidity functions, using UV-visible spectroscopy.36,37 Herein, p-nitroaniline was chosen as the basic indicator. IL and indicator were dissolved in water with concentrations of 50 and 0.1 mmol, respectively. Their Hammett acidity functions (H0) were calculated using the equation H0 = pKa(I)aq + log([I]/[IH+]) where pKa(I) is the pKa value of the p-nitroaniline (0.99) indicator solution, [I] and [IH+] are the molar concentrations of the unprotonated and protonated forms of the indicator, respectively.2.4 Glucose dehydration test
The catalytic activity experiments were performed in a 25 mL reaction vial that was heated in an oil-bath with a magnetic stirrer. In a standard experiment, CrCl3, cocatalyst (7–28 mol% to glucose or fructose) and substrate were dissolved in organic solvent (5 mL). Then the vial was placed in an oil-bath preheated to a certain temperature with stirring. At different time intervals, 50 μL samples were extracted and quenched immediately with deionized water (×100) for the product analysis.2.5 Product analysis
The products were analyzed by high performance liquid chromatography (HPLC) on an Agilent Alliance 1100 series chromatograph equipped with a refractive index detector and Shodex SURGER SP-0810 columns (300 mm × 8.0 mm). The mobile phase was ultrapure water at a flow rate of 0.7 mL min−1 and the column temperature was 70 °C. The concentrations of products were calculated based on the standard curve obtained with known concentrations of the standard substance.2.6 Density functional theory calculations
The geometries of all structures involved, including glucose, CrCl3, DMA, DMSO, the three anions (HSO4−, Cl− and Br−) and various complexes were optimized using density functional theory (DFT) with hybrid Becke 3-Lee–Yang–Parr (B3LYP)38,39 exchange–correlation functional using the Gaussian 03 program. The 6-311G(d,p) basis set was used for C, H, O, N, Cl and S atoms, while the Cr, Br atom was treated with the LanL2DZ basis set.40 In order to be consistent with the experiment conditions, all of the compounds were optimized using the polarizable continuum model (PCM) for DMA or DMSO at 393.15 K. Vibrational analyses on all optimized structures reveal a lack of imaginary frequencies, ensuring the presence of a true minimum. The interaction energies are also corrected by zero-point energy (ZPE). Various initial geometries of glucose were optimized and the most stable one was used for subsequent research. As for the study of reaction mechanism, DFT calculations were carried out to obtain energy barriers for reactants, intermediates (IMs), transition states (TSs) and products by the same method described above and to get minima in potential energy surfaces corresponding to stable molecular species and saddle points that corresponded to transition states. The intrinsic reaction coordinate (IRC) pathways have been traced in order to verify that the saddle point links two desired minima.3. Results and discussions
3.1 Glucose dehydration in different organic solvents
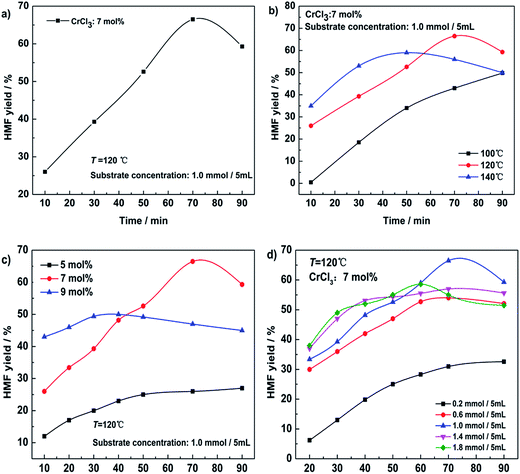
A set of glucose dehydration experiments were performed in DMF, DMA, DMSO and n-butyl alcohol over CrCl3 at 120 °C. The results are summarized in Fig. 2. HMF is obtained in moderate yield from glucose in DMA and n-butyl alcohol. More unidentified by-products were formed in DMF. In DMSO, less glucose was converted even if the time was prolonged (Fig. 2b). So we assume that the DMSO prevented CrCl3 from attacking glucose in some way. It is not possible to adjust the temperature of n-butyl alcohol over a wide range because of its low boiling point. So DMA was chosen as the solvent for the following research.Time, temperature, catalyst loading and substrate concentration are key factors for chemical reactions. Fig. 3 shows that the yield of HMF reaches its highest at 120 °C for 70 min with 7 mol% CrCl3 when the glucose concentration is 1.0 mmol/5 mL. The further research was performed under the optimal conditions.
3.2 Glucose dehydration with different Brønsted acids as cocatalyst in DMA
The IL is considered as not only a solvent but also a cocatalyst which may have synergy with the metal chloride for the conversion of glucose.20 So in this work, we chose three kinds of ILs ([NMP]HSO4, [NMP]Cl, [NMP]Br) which possessed the same cation and different anions to investigate their effect on HMF yield in DMA. Fig. 4a shows that addition of [NMP]HSO4 (7 mol% to glucose) lowers the yield of HMF slightly. What’s more, as the dosage increases, HMF yield decreases significantly (Fig. 4b). [NMP]Cl and [NMP]Br both accelerate HMF generation but don’t increase the highest yield. To confirm the crucial role of anions, H2SO4, HCl and HBr were chosen to replace the corresponding ILs (Fig. 4c and d). In a similar way HMF yield decreases as H2SO4 dosage increases and the reaction is promoted by HCl and HBr but without the HMF yield increasing. | ||
Fig. 4 Effect of different Brønsted acids on HMF yield. Conditions: DMA (5 mL), glucose (1 mmol), CrCl3 (7 mol%), CrCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Brønsted acids = 7 Brønsted acids = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 or 7 7 or 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 (mol mol−1), T = 120 °C. 28 (mol mol−1), T = 120 °C. | ||
3.3 Exploration of mechanism of action of the catalysts under different experimental conditions
In order to reveal why little HMF was obtained with addition of [NMP]HSO4 or H2SO4, we paid attention to the conversion of glucose. As can be seen from Fig. 5, glucose conversion reduces with increasing concentration of [NMP]HSO4 or H2SO4. So we inferred that HSO4− lowered the activity of CrCl3. | ||
Fig. 5 Effect of [NMP]HSO4 and H2SO4 on glucose conversion. Conditions: DMA (5 mL), glucose (1 mmol), CrCl3 (7 mol%), CrCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Brønsted acids = 7 Brønsted acids = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 or 7 7 or 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 (mol mol−1), T = 120 °C. 28 (mol mol−1), T = 120 °C. | ||
The mechanism for glucose dehydration to HMF involves fructose formation as a crucial reaction intermediate.25 So the question is which step do [NMP]Br, [NMP]Cl, HBr and HCl promote, accelerating the generation of HMF? To answer the question, a series of reactions were carried out with glucose and fructose as substrate, respectively. As is shown in Table 1, no HMF formed from glucose while high HMF yield was obtained from fructose over Brønsted acids. So we confirm Brønsted acids can’t realize the isomerization of glucose but mainly convert fructose to HMF. In addition, [NMP]Br and HBr show higher selectivity than [NMP]Cl and HCl, which is consistent with Binder’s treatise.41 While CrCl3 can catalyze both glucose isomerization and fructose dehydration to HMF. But compared with Brønsted acids, it takes a longer time for CrCl3 (7 mol%) to reach an equal yield of HMF from fructose. That’s why a certain amount of Brønsted acid accelerates HMF formation from glucose (Fig. 4).
| Substrate | Catalyst/mol% | t/min | T/°C | Conversion/% | HMF yield/% |
|---|---|---|---|---|---|
| a Conditions: DMA (5 mL), glucose or fructose (1 mmol), T = 120 °C. | |||||
| Glucose | [NMP]Cl (7) | 60 | 120 | 37.7 | 0 |
| Glucose | [NMP]Br (7) | 60 | 120 | 54.1 | 0 |
| Glucose | HCl (7) | 60 | 120 | 43.2 | 0 |
| Glucose | HBr (7) | 60 | 120 | 60.4 | 0 |
| Glucose | CrCl3 (7) | 60 | 120 | 93.5 | 59.6 |
| Fructose | [NMP]Cl (28) | 5 | 120 | 97.0 | 60.5 |
| Fructose | [NMP]Br (28) | 5 | 120 | 98.7 | 80.2 |
| Fructose | HCl (7) | 5 | 120 | 95.6 | 59.4 |
| Fructose | HBr (7) | 5 | 120 | 98.3 | 70.4 |
| Fructose | CrCl3 (7) | 5 | 120 | 94.9 | 10.2 |
| Fructose | [NMP]Cl (28) | 40 | 120 | <100 | 55.3 |
| Fructose | [NMP]Br (28) | 40 | 120 | <100 | 74.9 |
| Fructose | [NMP]HSO4 (28) | 40 | 120 | <100 | 40.3 |
| Fructose | HCl (7) | 40 | 120 | <100 | 71.1 |
| Fructose | HBr (7) | 40 | 120 | <100 | 74.2 |
| Fructose | H2SO4 (7) | 40 | 120 | <100 | 41.0 |
| Fructose | CrCl3 (7) | 40 | 120 | 96.1 | 67.8 |
3.4 Determination of H0 values of Brønsted acidic ILs
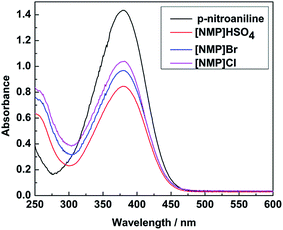
Due to the fact that dehydration of fructose is associated with the acidity of the catalyst, the acidic scale of these Brønsted acidic ILs was investigated with a basic indicator. The maximal absorbance of the unprotonated form of the indicator was observed at 379 nm in H2O. When an acidic IL was added, the absorbance of the unprotonated form of the indicator decreased. As shown in Fig. 6 the absorbance of the unprotonated form of the indicator on three acidic ILs decreased as follows: [NMP]Cl > [NMP]Br > [NMP]HSO4. Ultimately, we obtained the acidity order of the three ILs with the following H0 values (Table 2): [NMP]HSO4 (1.15) > [NMP]Br (1.31) > [NMP]Cl (1.41). Table 1 shows selectivity from fructose to HMF decreasing in the order [NMP]Br > [NMP]Cl > [NMP]HSO4. This implies that too strong acidity isn’t beneficial for fructose dehydration to HMF.| ILs | Amax | [I]/% | [IH+]/% | H0 |
|---|---|---|---|---|
| a Indicator: 4-nitroaniline; [I] and [IH+]: the unprotonated and protonated forms of the indicator; H0 = pKa(I)aq + log([I]/[IH+]), pKa(I)aq = 0.99. Solvent: deionized water; [IL] = 50 mmol L−1; [4-nitroaniline] = 0.1 mmol L−1; T = 20 °C. | ||||
| — | 1.435 | 100 | 0 | — |
| [NMP]HSO4 | 0.847 | 59.02 | 40.98 | 1.15 |
| [NMP]Br | 0.968 | 67.46 | 32.54 | 1.31 |
| [NMP]Cl | 1.038 | 72.33 | 27.67 | 1.41 |
3.5 Investigation of the mechanism of influence of solvents and Brønsted acids using a computational method
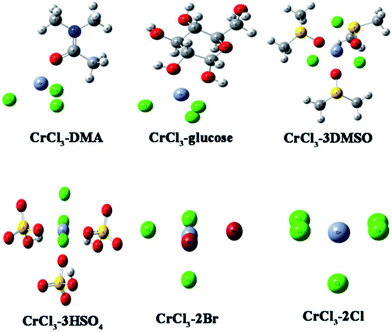
How do DMSO, [NMP]HSO4 and H2SO4 suppress glucose conversion? Does, just as Binder and Raines conjectured, a halide particularly bromide not only promote selective transformation of fructose but also serve as a ligand for chromium?41 Do [NMP]Br, [NMP]Cl, HBr and HCl improve the reactivity of CrCl3, promoting the glucose to fructose isomerization step that determines the overall HMF selectivity? To solve these problems, a computational method was employed.Binder et al. have reported that in DMA–LiCl solvent, lithium ions could associate with DMA to form DMA–Li+,41 Hensen and co-workers confirmed in an MeCl2/[EMIM]Cl (Me = Cr, Cu and Fe) system, that [MeCl4]2− complexes were the reactive species in the MeCl2-catalyzed glucose dehydration by a combination of kinetic experiments and density functional theory (DFT) calculations.28 So in our work, the structural and coordination properties of complexes formed upon the interaction of chromium(III) chlorides with solvent, ionic liquids and glucose were first studied by density functional theory (DFT) calculations (Table 3). The smaller the value of ΔG393.15K is, the easier the corresponding species forms. Entry 1–4 in Table 3 represent the DMA–CrCl3–glucose system. There is no obvious difference in values of ΔG393.15K. That is to say, CrCl3 can combine with both glucose and DMA. When combining with DMA, CrCl3–DMA is the most thermodynamically favorable. In other words, the reaction of glucose can be catalyzed by CrCl3 and CrCl3–DMA in DMA. While in the DMSO–CrCl3–glucose system (Entry 5–8), the interaction of the CrCl3 with DMSO is strongly favorable (ΔG393.15K = −114.56 kJ mol−1) and leads to the formation of a six-coordinated structure. From the configuration of CrCl3–3DMSO in Fig. 7 we can see the Cr center is surrounded completely by DMSO and Cl−, impeding its combination with the substrate and restraining the conversion of glucose. That’s why DMSO isn’t appropriate for glucose conversion catalyzed by CrCl3. Similarly, in the DMA–glucose–CrCl3–[NMP]HSO4 or H2SO4 system (Entry 9–12), CrCl3 mainly combines with HSO4−, forming CrCl3–3HSO4 (ΔG393.15K = −78.61 kJ mol−1). When the catalyst dosage is CrCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [NMP]HSO4 = 7
[NMP]HSO4 = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (mol mol−1), the amount of HSO4− isn’t enough to combine with CrCl3 to form more CrCl3–3HSO4, resulting in only a slight decrease of glucose conversion. As the molar ratio of [NMP]HSO4 and CrCl3 increases, more and more Cr centers are surrounded, leading to an obvious decrease of glucose conversion. While combination of CrCl3 and cation of IL is thermodynamically unfavorable (ΔG393.15K = 70.69 kJ mol−1), just as described in the literature,29 cations provide an efficient charge-compensating environment for the anionic metal chloride complexes. No coordination of the cation to metal centers is observed. So in order to simplify the simulation, the cation of IL was not considered during the next part of the mechanism research.
7 (mol mol−1), the amount of HSO4− isn’t enough to combine with CrCl3 to form more CrCl3–3HSO4, resulting in only a slight decrease of glucose conversion. As the molar ratio of [NMP]HSO4 and CrCl3 increases, more and more Cr centers are surrounded, leading to an obvious decrease of glucose conversion. While combination of CrCl3 and cation of IL is thermodynamically unfavorable (ΔG393.15K = 70.69 kJ mol−1), just as described in the literature,29 cations provide an efficient charge-compensating environment for the anionic metal chloride complexes. No coordination of the cation to metal centers is observed. So in order to simplify the simulation, the cation of IL was not considered during the next part of the mechanism research.
| Entry | Solvent | Ligand | Species of CrCl3 | ΔG393.15K (kJ mol−1)b |
|---|---|---|---|---|
| a Optimizations and frequencies were calculated at the B3LYP/6-311G(d,p) level using the polarizable continuum model (PCM) for DMA or DMSO.b ΔG393.15K = Gspecies/393.15k − GCrCl3/393.15k − nGDMA or DMSO (n = 1, 2, 3).c Cation of IL. | ||||
| 1 | DMA | DMA | CrCl3–DMA | −34.00 |
| 2 | DMA | DMA | CrCl3–2DMA | −31.72 |
| 3 | DMA | DMA | CrCl3–3DMA | −16.00 |
| 4 | DMA | Glucose | CrCl3–glucose | −26.62 |
| 5 | DMSO | DMSO | CrCl3–DMSO | −58.34 |
| 6 | DMSO | DMSO | CrCl3–2DMSO | −96.19 |
| 7 | DMSO | DMSO | CrCl3–3DMSO | −114.56 |
| 8 | DMSO | Glucose | CrCl3–glucose | −35.25 |
| 9 | DMA | NMPc | CrCl3–NMPc | 70.69 |
| 10 | DMA | HSO4− | CrCl3–HSO4 | −31.12 |
| 11 | DMA | HSO4− | CrCl3–2HSO4 | −38.13 |
| 12 | DMA | HSO4− | CrCl3–3HSO4 | −78.61 |
| 13 | DMA | Br− | CrCl3–Br | −67.8 |
| 14 | DMA | Br− | CrCl3–2Br | −93.6 |
| 15 | DMA | Br− | CrCl3–3Br | −81.4 |
| 16 | DMA | Cl− | CrCl3–Cl | −50.4 |
| 17 | DMA | Cl− | CrCl3–2Cl | −55.2 |
| 18 | DMA | Cl− | CrCl3–3Cl | −27.0 |
In DMA–glucose–CrCl3–[NMP]Br or HBr and DMA–glucose–CrCl3–[NMP]Cl or HCl systems (Entry 13–18), interactions of CrCl3 with glucose, DMA, and anion of ILs (Br, Cl) were compared. Just as Binder and Raines said, a halogen particularly bromine can combine with chromium facilely to form different complexes. The DFT calculations indicate that the five-coordinated structures are the dominant Cr3+-containing complexes after addition of Brønsted acid. What’s more, the resulting CrCl3–2Cl and CrCl3–2Br species adopt a hemispheric geometry (Fig. 7), exposing the Cr3+ center. As a result, this kind of five-coordinated structure can still catalyze glucose. But it’s still unknown whether Br− and Cl− improve the activity of CrCl3 for glucose isomerization. Herein a computational method was used to simulate the process over CrCl3, CrCl3–DMA, CrCl3–2Br and CrCl3–2Cl, which were the dominant species of CrCl3. What is illustrated is that the four complexes represent the DMA–glucose–CrCl3, DMA–glucose–CrCl3–[NMP]Br or HBr, DMA–glucose–CrCl3–[NMP]Cl or HCl systems, respectively.
According to the previous literature,31,42 a mechanism for the Cr3+ complex-catalyzed isomerization of glucose to fructose is proposed as shown in Scheme 1. The reaction is initiated by the direct coordination of glucose with the Lewis acidic Cr3+ center and deprotonation of the O1H moiety of the carbohydrate (intermediate 2). The subsequent protonation of O5 opens the glucopyranose ring and O2H is deprotonated at the same time (intermediate 4). What happens next is an H-shift from C2 to C1 which is the rate-determining step (TS-H-shift). Fructose is formed by protonation of intermediate 6 at the anionic O1 site and closure of the furanose ring. Based on the mechanism, optimized structures of reaction intermediates and transition-state of glucose isomerization to fructose over different complexes in DMA were obtained (see Section S3 of ESI†). The corresponding free energy diagrams at 393.15 K are shown in Fig. 8.
 | ||
| Scheme 1 Mechanism of glucose isomerization to fructose catalyzed by Cr3+ complex in DMA ([Cr3+] represents different complexes of Cr3+ and anions or DMA). | ||
 | ||
| Fig. 8 The DFT-computed free energy diagrams of CrCl3, CrCl3–DMA, CrCl3–2Br, CrCl3–2Cl catalyzed process. | ||
As is shown in Fig. 8, the free energy barrier for CrCl3 combining with glucose directly equals −26.6 kJ mol−1. When CrCl3 is coordinated by any other group especially Br− or Cl−, it takes a higher free energy barrier for the complex to attach to glucose. But based on the previous discussion, a negative effect of solvent or other groups is inevitable. So free energy diagrams of CrCl3–DMA, CrCl3–2Br, CrCl3–2Cl catalyzed process are compared (Fig. 8b). The process of H shifting from C2 to C1 is considered as the rate-controlling step in glucose isomerization.28 So the free energy barriers for this step are mainly discussed. The activation free energy barriers for H-shift decrease in the order of CrCl3–DMA (53.0 kJ mol−1) > CrCl3–2Br (47.9 kJ mol−1) > CrCl3–2Cl (41.7 kJ mol−1). This means CrCl3–2Br, CrCl3–2Cl promote migration of H slightly. However, the overall barriers increase by about 20 kJ mol−1. That is to say, Br− and Cl− can replace DMA to combine with Cr3+ in DMA, but they have not promoted glucose isomerization. So we speculate that only Brønsted acid catalyzes fructose dehydration to HMF in the system studied in our work.
4. Conclusions
HMF preparation from glucose in different organic solvents catalyzed by CrCl3 (catalyst) and Brønsted acid (cocatalyst) was carried out. Research was carried out to investigate why DMSO suppressed the generation of HMF and what was the role of Brønsted acid in the reaction using experimental and theoretical methods. When DMSO acted as solvent, CrCl3 preferentially combined with DMSO, forming six-coordinated structures causing the Cr3+ center to be surrounded completely, suppressing glucose conversion. While in DMA, CrCl3 could attach to glucose directly, catalyzing the conversion of glucose. At the same time, CrCl3 also coordinated with DMA, mainly forming four-coordinated structures. There were still exposed metal sites to catalyze glucose. The Brønsted acid is mainly responsible for fructose dehydration to HMF. The anion of the Brønsted acid can serve as a ligand for CrCl3, among which HSO4− can inactivate CrCl3 by surrounding it like DMSO. Br− and Cl− can also coordinate with CrCl3, mainly forming five-coordinated complexes which present hemispherical structures. In addition, these complexes don’t lower the overall barriers for glucose isomerization. So only Brønsted acid works in fructose dehydration to HMF. What is said above can be treated as a guide to choose solvent and cocatalysts for a Lewis acid or as guidance for the synthesis of a Lewis acid complex catalyst for cellulosic biomass conversion.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (21206057), the Natural Science Foundation of Jiangsu Province, China (BK2012118) and (BK2012547), and MOE & SAFEA for the 111 Project (B13025) for financial support.References
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick Jr, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- M. Bicker, J. Hirth and H. Vogel, Green Chem., 2003, 5, 280–284 RSC.
- Y. Roman-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef CAS PubMed.
- X. Qi, M. Watanabe, T. M. Aida and J. R. L. Smith, Green Chem., 2008, 10, 799–805 RSC.
- X. Tong and Y. Li, ChemSusChem, 2010, 3, 350–355 CrossRef CAS PubMed.
- C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165–169 CrossRef CAS PubMed.
- J. He, Y. Zhang and E. Y. Chen, ChemSusChem, 2013, 6, 61–64 CrossRef CAS PubMed.
- L. Wu, J. Song, B. Zhang, B. Zhou, H. Zhou, H. Fan, Y. Yang and B. Han, Green Chem., 2014, 16, 3935–3941 RSC.
- B. Liu, Z. Zhang and Z. K. Zhao, Chem. Eng. J., 2013, 215–216, 517–521 CrossRef CAS PubMed.
- L. Zhou, R. Liang, Z. Ma, T. Wu and Y. Wu, Bioresour. Technol., 2013, 129, 450–455 CrossRef CAS PubMed.
- S. Xiao, B. Liu, Y. Wang, Z. Fang and Z. Zhang, Bioresour. Technol., 2014, 151, 361–366 CrossRef CAS PubMed.
- F. Tao, H. Song and L. Chou, RSC Adv., 2011, 1, 672–676 RSC.
- Z. Zhang, B. Liu and Z. Zhao, Carbohydr. Polym., 2012, 88, 891–895 CrossRef CAS PubMed.
- J. A. Geboers, S. Van de Vyver, R. Ooms, B. Op de Beeck, P. A. Jacobs and B. F. Sels, Catal. Sci. Technol., 2011, 1, 714–726 Search PubMed.
- Z. C. Zhang, WIREs Energy Environ., 2013, 2, 655–672 CrossRef CAS.
- K. Beckerle and J. Okuda, J. Mol. Catal. A: Chem., 2012, 356, 158–164 CrossRef CAS PubMed.
- E. F. Dunn, D. Liu and E. Y. X. Chen, Appl. Catal., A, 2013, 460–461, 1–7 CrossRef CAS PubMed.
- J. Song, B. Zhang, J. Shi, H. Fan, J. Ma, Y. Yang and B. Han, RSC Adv., 2013, 3, 20085–20090 RSC.
- V. Choudhary, S. H. Mushrif, C. Ho, A. Anderko, V. Nikolakis, N. S. Marinkovic, A. I. Frenkel, S. I. Sandler and D. G. Vlachos, J. Am. Chem. Soc., 2013, 135, 3997–4006 CrossRef CAS PubMed.
- S. Hu, Z. Zhang, J. Song, Y. Zhou and B. Han, Green Chem., 2009, 11, 1746–1749 RSC.
- Y. Qu, C. Huang, Y. Song, J. Zhang and B. Chen, Bioresour. Technol., 2012, 121, 462–466 CrossRef CAS PubMed.
- S. Saravanamurugan, M. Paniagua, J. A. Melero and A. Riisager, J. Am. Chem. Soc., 2013, 135, 5246–5249 CrossRef CAS PubMed.
- J. Wang, J. Ren, X. Liu, J. Xi, Q. Xia, Y. Zu, G. Lu and Y. Wang, Green Chem., 2012, 14, 2506–2512 RSC.
- K. Nakajima, R. Noma, M. Kitano and M. Hara, J. Mol. Catal. A: Chem., 2014, 388–389, 100–105 CrossRef CAS PubMed.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS PubMed.
- G. Tsilomelekis, T. R. Josephson, V. Nikolakis and S. Caratzoulas, ChemSusChem, 2014, 7, 117–126 CrossRef CAS PubMed.
- X. Qian, J. Phys. Chem. A, 2011, 115, 11740–11748 CrossRef CAS PubMed.
- E. A. Pidko, V. Degirmenci, R. A. van Santen and E. J. M. Hensen, Angew. Chem., Int. Ed., 2010, 49, 2530–2534 CrossRef CAS PubMed.
- E. A. Pidko, V. Degirmenci, R. A. van Santen and E. J. Hensen, Inorg. Chem., 2010, 49, 10081–10091 CrossRef CAS PubMed.
- Y. Zhang, E. A. Pidko and E. J. Hensen, Chemistry, 2011, 17, 5281–5288 CrossRef CAS PubMed.
- E. A. Pidko, V. Degirmenci and E. J. M. Hensen, ChemCatChem, 2012, 4, 1263–1271 CrossRef CAS.
- J. Guan, Q. Cao, X. Guo and X. Mu, Comput. Theor. Chem., 2011, 963, 453–462 CrossRef CAS PubMed.
- L. Zhang, M. Xian, Y. He, L. Li, J. Yang, S. Yu and X. Xu, Bioresour. Technol., 2009, 100, 4368–4373 CrossRef CAS PubMed.
- H. R. Shaterian and M. Ranjbar, J. Mol. Liq., 2011, 160, 40–49 CrossRef CAS PubMed.
- X. U. Junming, J. Jianchun, Z. Zhiyue and L. Jing, Process Saf. Environ. Prot., 2010, 88, 28–30 CrossRef PubMed.
- D. A. Kotadia and S. S. Soni, Catal. Sci. Technol., 2013, 3, 469–474 CAS.
- Y. Wang, D. Jiang and L. Dai, Catal. Commun., 2008, 9, 2475–2480 CrossRef CAS PubMed.
- M. N. Glukhovtsev, R. D. Bach and C. J. Nagel, J. Phys. Chem. A, 1997, 101, 316–323 CrossRef CAS.
- C. Adamo and V. Barone, Chem. Phys. Lett., 1997, 274, 242–250 CrossRef CAS.
- Q. Meng, F. Wang and M. Li, J. Mol. Model., 2012, 18, 4955–4963 CrossRef CAS PubMed.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- G. Yang, E. A. Pidko and E. J. Hensen, ChemSusChem, 2013, 6, 1688–1696 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra02057c |
| This journal is © The Royal Society of Chemistry 2015 |