Conductive carbon nanotube paper by recycling waste paper
Do-Hyun Kim*a,
Yong-In Choa,
Jun Hee Choia,
Hag-Soo Kimb,
Hyun Chang Shinc,
Tong Sun Leec,
Jin Won Jungc,
Hong-Dae Kimd,
Dong-Jin Leee and
Gyu Tae Kim*a
aSchool of Electrical Engineering, Korea University, Anam-dong, Seongbuk-gu, Seoul 136-713, Republic of Korea. E-mail: nanotube@korea.ac.kr; Tel: +82-2-3290-3801
bDepartment of Chemical Engineering, The University of Seoul, 163 Seoulsiripdaero (90 Jeonnong-dong), Dongdaemun-gu, Seoul 130-743, Republic of Korea
cENP Co., Ltd., 712-1, 27, Doonchondaelo 457gil, Jungwon-gu, Seongnam-si, Gyeonggi-do 462-806, Republic of Korea
dGreen Technology Center, Korea Institute of Industrial Technology, 385-1 Dajeon, Jung-gu, Ulsan 681-310, Republic of Korea
eNew Functional Components Research Team, Korea Institute of Footware & Leather Technology, 152 Danggamseo-ro, Busanjin-gu, Busan 614-100, Republic of Korea
First published on 27th March 2015
Abstract
This paper reports on an easy process of fabricating conductive carbon nanotube (CNT) paper by recycling waste paper. In the absence of a dispersion agent, we just mixed and ground multi-walled carbon nanotubes (MWCNT) and shredded A4 paper together in water. CNT paper was obtained after filtering and rolling the mixture of MWCNT and waste paper. The thickness of the CNT paper was controllable by using different volumes of the mixture in the fabrication. Scanning electron microscope images showed that MWCNT was evenly stuck to the surface of the cellulose. Raman spectra indicated that the structure of a sidewall of MWCNT was influenced by cellulose through the mixing process. In addition, the thermal stability of CNT paper was enhanced in proportion to the amount of MWCNT used. The presence of MWCNT also contributed to the enhancement in the elastic properties of CNT paper. Depending on the amount of MWCNT and the thickness of CNT paper, the sheet resistance of CNT paper varied from 49.1 to 3365.6 Ω sq−1. This CNT paper can be utilized in the fields of electronic devices and energy storage devices, leading to easy mass-production at low cost.
Introduction
For more than 4000 years, paper has been mainly used for the purpose of recording information. With the advance in the fields of flexible displays and wearable devices, paper has recently been investigated as an alternative to substitute plastic substrates such as polyethylene terephthalate,1–3 polyethylene naphthalate,4–6 and polyimide.7–9 Furthermore, paper becomes more useful in the application to electronics when it has electrical conductivity. There have been efforts to fabricate paper having electrical conductivity.The fabrication method of conductive paper can be classified into two ways. One is to make a composite of a conductive material and cellulose fiber by using a typical process of paper fabrication.10–15 As a filler to make paper conductive, carbon black,10 carbon fiber,10,11 graphite, and carbon nanotube (CNT)12–15 were adapted recently. The other is to coat paper with conductive materials by using conductive ink16,17 as well as conductive polymer.18 In both methods, CNT is utilized as a material to make paper electrically conductive because CNT has superior electrical conductivity. Hu et al. fabricated a highly conductive paper by coating CNT ink on paper.16 The sheet resistance of the conductive paper was controlled in the range from 105 to 10 Ω sq−1 by varying the thickness of paper. As a modified method, cellulose sheet was dipped in a solution where CNT is dispersed evenly, and conductive paper was successfully obtained.19
In spite of using CNT as superior conducting filler, there is still a technical barrier to be improved. In preparing conductive paper by the method of mixing CNT and cellulose, we should conduct a typical procedure of obtaining cellulose fiber by chemical modification of pulp, which is a cost and time consuming process. In addition, the use of a surfactant is inevitable to achieve good dispersion of CNT solution. Meanwhile, coating CNT on paper is relatively easier than the typical method mentioned above, but we cannot avoid preparing an ink containing conductive material. This is another kind of a laborious task. In this case, the use of a surfactant cannot be also avoided in the step of conductive ink formulation when CNT is involved. In any case, the process to remove the surfactant is required to make paper conductive. Detailed reason can be explained as following: generally, paper becomes electrically conductive due to CNT which is continuously connected as a structure of networks on paper. We can expect electrical conductivity from the percolated CNT networks constituting electrical paths in paper. However, CNT networks do not play a role as an electrical path in the presence of a surfactant since CNT is covered with the surfactant which is an insulator electrically. Therefore, post-process is required to remove the surfactant from conductive paper in all cases.20 Furthermore, the chemical procedure of obtaining cellulose from pulp should be avoided in the fabrication of conductive paper if possible.
To overcome this problem, we show a simple way of preparing conductive paper by using CNT and waste paper without a surfactant. To evade the chemical treatment to obtain cellulose fiber, we used waste paper (shredded A4 paper) as a source of cellulose for the fabrication of conductive paper. This method is attractive in the respect of recycling waste paper as well as avoiding a chemical treatment occurred in the fabrication of paper. And, multi-walled carbon nanotube (MWCNT) was employed as a material to make paper electrically conductive. Our idea is to mechanically grind and mix shredded paper and MWCNT together in water to obtain the slurry of cellulose and MWCNT. Through the mechanical mixing, we can expect good dispersion of MWCNT in the slurry without using a surfactant. After the mixing, conductive paper is achieved via filtering and rolling the slurry. By varying the amount of MWCNT and the volume of the slurry, the electrical conductivity and thickness of conductive paper was controllable.
Apart from mass production, our method is time- and cost-effective compared with the mentioned methods of fabricating conductive paper. We think that CNT paper can be applied to diverse fields of electrode of energy storage device, flame retard, and electromagnetic shielding as well as flexible substrate.
Experimental
Preparation of CNT paper
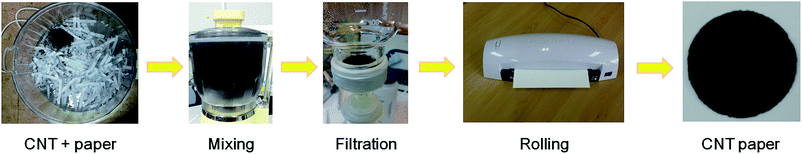
Fig. 1 shows the process of fabricating conductive paper by recycling waste paper and CNT. As shown in Fig. 1, we used shredded A4 paper as a source of cellulose and MWCNT (Nanocyl NC 7000, average diameter: 9.5 nm, average length: 1.5 μm, purity: 90%, surface area: 250–300 m2 g−1) as a conductive material. A kitchen mixer grinder was used to obtain the slurry of MWCNT and cellulose as shown in the mixing process of Fig. 1. MWCNT and 1.5 g of shredded paper were added in 500 ml of de-ionized water and mixed for 4 min at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. To control the electrical conductivity of paper, a different amount of MWCNT (3, 7, and 10 wt%) was added in the preparation of the slurry. After mixing, the slurry was filtered using a filter paper (ADVANTEC, 5C, diameter: 5.5 cm) by vacuum and washed with 100 ml of de-ionized water to remove the unknown chemicals from waste paper. In this step, various volumes of the slurry (10, 15, and 20 ml) were used to fabricate paper having different thickness. Next, the filtered slurry was dried at 120 °C and pressed by office laminator to obtain paper having even and smooth surface. As a reference sample, we prepared paper by using 20 ml of the slurry without MWCNT (hereafter, reference).
000 rpm. To control the electrical conductivity of paper, a different amount of MWCNT (3, 7, and 10 wt%) was added in the preparation of the slurry. After mixing, the slurry was filtered using a filter paper (ADVANTEC, 5C, diameter: 5.5 cm) by vacuum and washed with 100 ml of de-ionized water to remove the unknown chemicals from waste paper. In this step, various volumes of the slurry (10, 15, and 20 ml) were used to fabricate paper having different thickness. Next, the filtered slurry was dried at 120 °C and pressed by office laminator to obtain paper having even and smooth surface. As a reference sample, we prepared paper by using 20 ml of the slurry without MWCNT (hereafter, reference).
Characterization
Scanning electron microscope (SEM, MIRA 3 FEG SEM) was employed to observe the surface of CNT paper and the morphology between MWCNT and cellulose. The presence of MWCNT in the paper was confirmed by Raman spectroscopy (HORIBA Jobin-Yvon, HR-800UV, Ar laser, 514.5 nm). To investigate the thermal stability of CNT paper, thermogravimetric analysis (TGA, TA Instruments, Q500) was conducted. Temperature was raised up to 450 °C at a 10 °C min−1 ramp rate. To study the elastic properties of CNT paper, Young's modulus was measured with a universal test machine (Instron 5582) at a cross-head speed of 1 mm min−1. By using 4-point probe method (Advanced Instrument Technology, CMT-series), the sheet resistance of CNT paper was measured at six different sites of each paper and the average value was used as sheet resistance of the CNT paper. By using digital thickness gage (Mitutoyo Corp.), the thickness of CNT paper was measured in the same way as performed in measuring sheet resistance. To show that CNT paper is reproducible, three sheets of CNT paper were used in the analysis of sheet resistance and thickness.CNT paper which was prepared by using 20 ml of the slurry at the concentration of 3, 7, and 10 wt% was used in the analysis for SEM, Raman spectroscopy, and TGA (hereafter, 3, 7, and 10%). For the measurement of Young's modulus, 200 ml of the slurry at the concentration of 3, 7, and 10 wt% was filtered by using Buchner funnel with a filter paper (CHMLAB GROUP, no. F2040-110, diameter: 11 cm) to obtain a large size of CNT paper.
Results and discussion
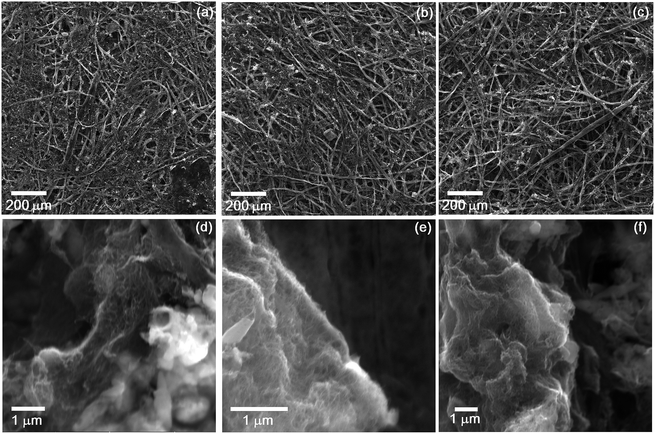
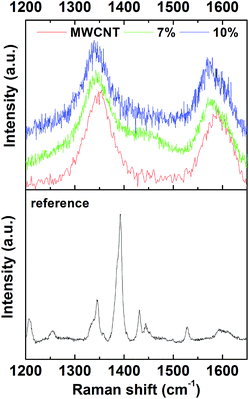
We conducted SEM analysis to observe the morphology of cellulose and MWCNT in CNT paper. Fig. 2 represents the SEM images of 3, 7, and 10% prepared by using 20 ml of the slurry. The observation was conducted at low (Fig. 2a–c) and high (Fig. 2d–f) magnification, respectively. The images seen in Fig. 2a–c present the morphology of cellulose fibers observed in the surface of each paper. We can see from the images that cellulose fibers are tangled together in all cases, meaning that our method of grinding and filtration was successful in fabricating paper. Despite we used shredded paper as a cellulose source, the morphology of a tangled structure is consistent with that of other works on paper fabrication.10–13,16–19,21 In order to see the morphology between cellulose and MWCNT, we observed CNT paper at high magnification. Fig. 2d–f represent the images obtained 3, 7, and 10% CNT paper, respectively. From Fig. 2d, we can notice that MWCNT exists in the surface of cellulose even in a small amount. When the weight percentage of MWCNT increased up to 7 and 10% in CNT paper, more MWCNTs were observed in the surface of cellulose as seen in Fig. 2e and f. Interestingly, the SEM images confirm that MWCNT is evenly dispersed on the surface of cellulose without using dispersion agent in the process of the mixing. This means that the mechanical mixing of shredded paper and MWCNT in water is significant to good dispersion of MWCNT in the surface of cellulose. In other words, the mechanical mixing can substitute the usage of dispersion agent for MWCNT in the preparation of conductive paper. We think that the morphological similarity between MWCNT and cellulose also contributed to a well-woven structure of CNT paper.Raman spectroscopy is a useful way to detect the existence of MWCNT because it is sensitive in the Raman shift range around 1350 and 1590 cm−1. Furthermore, the effect of cellulose on MWCNT can be analysed by inspecting a change of spectra in the ranges. Therefore, we conducted Raman spectroscopic analysis on CNT paper to clarify the presence of MWCNT and the mutual influence between cellulose and MWCNT. Fig. 3 exhibits the Raman spectra measured from reference, 3, 7, and 10%. To confirm the Raman spectra originated from cellulose, we obtained the spectra from reference paper. The result was displayed in the bottom of Fig. 3. As shown in the figure, several spectra are detected from the reference paper and the strongest intensity appears at 1392 cm−1. Then, the Raman spectra of MWCNT were measured as displayed in the top of Fig. 3. We can see two major spectra at 1374 and 1591 cm−1. The peak at 1374 cm−1 has been known as D-band which is related to defects or disorder in CNT. Meanwhile, G-band, originated from sp2-hybridized carbon in honeycomb structure, has been reported to appear around 1590 cm−1. In the case of 3%, we detected only the spectra of cellulose than the ones corresponding to MWCNT even though the paper showed a grey colour. This means that 3 wt% is not the concentration enough to be detected in Raman spectroscopy analysis although we observed MWCNT in SEM analysis. In the case of 7%, we can see that D- and G-band spectra appear at 1343 and 1570 cm−1, respectively. And, no spectra are observed around 1392 cm−1 where the reference paper shows the strongest intensity in the measured Raman spectra. In addition, the presence of cellulose shifted D- and G-band spectra to the left, compared with the spectra measured from MWCNT. The same phenomenon occurs in the case of 10%. Both D- and G-band are shifted to the left and positioned at 1339 and 1570 cm−1, respectively. According to the review on Raman spectra of CNT by Dresselhaus and co-workers,22 the shift of G-band spectra breaks out when structural modification occurs in a sidewall of MWCNT by the attachment of other chemical species. Therefore, our result indicates a possibility that the structure of a sidewall of MWCNT has been modified by mixing MWCNT with cellulose together. Also, this is supported by our SEM analysis showing that MWCNT was attached to the surface of cellulose.
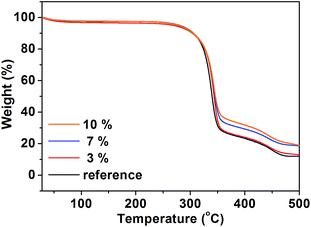
MWCNT has been known to show superior properties thermally and mechanically. Hence, the presence of MWCNT in paper can enhance its thermal stability, depending on the concentration of MWCNT. Therefore, TGA analysis was conducted to investigate the thermal stability of CNT paper by the amount of MWCNT. Fig. 4 gives weight loss with respect to temperature of reference, 3, 7, and 10%, respectively. The previous study reported that paper slightly lost a weight below 100 °C due to water and that a weight loss by the thermal decomposition of cellulose occurred drastically above 300 °C.23 The same phenomenon appears in Fig. 4, irrespective of the presence of MWCNT. And, all samples show a steep decrease in weight loss when temperature is higher than 300 °C. Then, the thermal decomposition proceeds slowly as the temperature reaches to 350 °C. This result is also consistent with the result of the work mentioned above. However, we can see that all samples have a different decomposition rate at 350 °C, depending on the amount of MWCNT in CNT paper. Reference and 3% show a similar behaviour in weight loss above 350 °C. Meanwhile, 7 and 10% result in a less weight loss at 350 °C, meaning that the increased content of MWCNT contributed to the enhancement of thermal stability of CNT paper. We can notice that 10% exhibits the strongest thermal stability among the prepared samples. From this, we can deduce that the thermal stability of CNT paper can be enhanced significantly when the concentration of MWCNT is at least 7%.
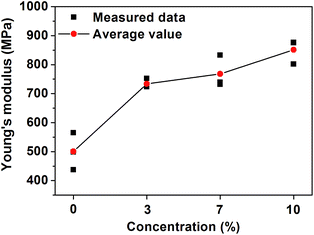
To see the effect of the presence of CNT on the elastic properties of CNT paper, we measured Young's modulus of each CNT paper as well as reference. Three sheets of paper were used for the measurement, and the results were plotted in Fig. 5. The figure displays the average Young's modulus (red circle) of the measured data and raw data (black square). As shown in Fig. 5, reference has the average value of 500.4 MPa and Young's modulus drastically increased up to 733.8 MPa with the addition of MWCNT. When the concentration of MWCNT is 7%, Young's modulus of CNT paper was enhanced from 733.8 to 768.0 MPa. And, the maximum value of 851.2 MPa was obtained as the concentration increased up to 10%. This means that the presence of CNT enhances the elastic properties of CNT paper in proportion to the concentration.
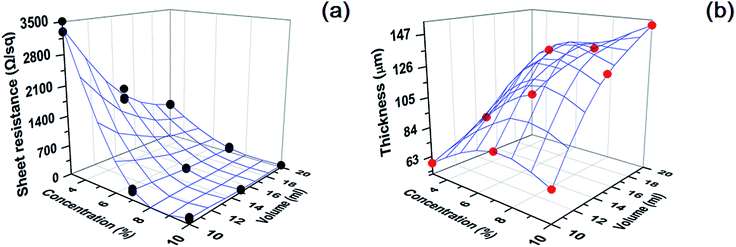
By varying the volume of the slurry and the amount of MWCNT, CNT paper can have different thickness and electrical conductivity. To clarify the influence of the MWCNT concentration and thickness on electrical conductivity, we prepared conductive paper which has different thickness and electrical conductivity, and measured the thickness and sheet resistance of CNT paper. Fig. 6a represents a plot of the sheet resistance of conductive paper versus the concentration of MWCNT and the volume of the slurry. As shown in the figure, we can notice that the sheet resistance gradually decreases as the concentration increases in CNT paper. The sheet resistance is varying from 49.1 to 3365.6 Ω sq−1, depending on the amount of MWCNT. This means that the conductivity of paper can be controlled by varying the concentration of MWCNT. This also points out that MWCNTs are networked quite well in the paper, providing an electrical path for electron. Fig. 6a shows the effect of volume of the slurry on the sheet resistance. When the concentration is fixed to 3 wt%, the sheet resistance of CNT paper drastically decreases with the increase in the slurry volume. Meanwhile, the trend of a sudden decrease in the sheet resistance does not appear at the concentration of 7 and 10% in spite of the increased volume of the slurry. This means that 7 and 10% are the proper concentration constituting electrical path in paper at the small volume of the slurry, leading to low sheet resistance. On the other hand, 3% is the concentration influenced by the volume of the slurry because the sheet resistance decreases with respect to the increase of the volume. That is, we cannot expect enough electrical paths by CNT network when the paper is prepared by 10 ml of the slurry at 3 wt%. However, the increase of the slurry volume provides more electrical paths as a form of CNT networks in the paper, resulting in the decrease of the sheet resistance from 3365.6 to 895.5 Ω sq−1. Fig. 6b presents a change of the thickness with respect to the CNT concentration and the slurry volume. The figure reveals that regardless of CNT concentration, the thickness of CNT paper becomes thick in proportion to the volume of the slurry. The maximum and minimum in the thickness of paper are 151.7 and 58.1 μm, respectively. From Fig. 6b, we can notice that the concentration of CNT contained in paper does not influence the thickness of paper.
We folded origami rose to show the CNT paper is foldable without losing electrical conductivity. Fig. 7a indicates the CNT paper prepared with different concentrations of MWCNT based on shredded paper. The image reveals that the colour of paper becomes close to black with the increase of MWCNT concentration. In all cases, MWCNT was not stuck to hands when we treated the CNT paper, meaning that MWCNT was captured well whining paper as seen in SEM images. However, when the concentration increased to 15 wt%, MWCNT was stuck to hands in touching and folding CNT paper. This is the reason why we employed 10 wt% of MWCNT as the maximum concentration in the fabrication of CNT paper. As mentioned before, the thickness of CNT paper can be controlled by changing the volume of the slurry. This means that we can easily prepare well-foldable CNT paper which keeps good electrical conductivity. Fig. 7b displays origami rose prepared by CNT paper, which plays a role as a part of circuit. This indicates that foldable and conductive paper can be prepared compatibly.
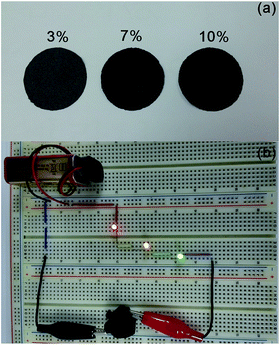 | ||
| Fig. 7 (a) CNT paper prepared by using 20 ml of the slurry and (b) origami rose connected to the circuit. CNT paper is foldable and conductive compatibly as shown in the figure. | ||
Conclusions
We successfully demonstrated the method to fabricate conductive CNT paper by grinding and mixing shredded paper and MWCNT in water. Without using a surfactant, the mechanical grinding led to even dispersion of MWCNT on the surface of cellulose. The presence of MWCNT in paper enhanced the thermal stability of CNT paper and shifted the G-band spectra of Raman spectroscopy to the left in the Raman shift. The sheet resistance and thickness of CNT paper were controllable by varying the amount of MWCNT and the volume of the slurry in the step of the paper fabrication. The maximum and minimum sheet resistance were 3365.6 and 49.1 Ω sq−1, respectively. In addition, the thickness of CNT paper was controlled from 58.1 to 151.7 μm.We think that this work will open a potential avenue to mass production of conductive paper as well as recycle of waste resource.
Acknowledgements
This work was supported by BK21 Plus Humanware Information Technology, the Center for Advanced Soft Electronics under the Global Frontier Research Program of the Ministry of Education (NRF-2014M3A6A5060942).References
- D. Kong, L. T. Le, Y. Li, J. L. Zunino and W. Lee, Langmuir, 2012, 28, 13467 CrossRef CAS PubMed.
- B. J. Kim, H. Jang, S.-K. Lee, B. H. Hong, J.-H. Ahn and J. H. Cho, Nano Lett., 2010, 10, 3464 CrossRef CAS PubMed.
- L. Huang, Y. Huang, J. Liang, X. Wan and Y. Chen, Nano Res., 2011, 4, 675 CrossRef CAS.
- T. Sekitani, Y. Kato, S. Iba, H. Shinaoka, T. Someya, T. Sakurai and S. Takagi, Appl. Phys. Lett., 2005, 86, 073511 CrossRef PubMed.
- N. Petrone, I. Meric, J. Hone and K. L. Shepard, Nano Lett., 2012, 13, 121 CrossRef PubMed.
- S. Borini, R. White, D. Wei, M. Astley, S. Haque, E. Spigone, N. Harris, J. Kivioja and T. Ryhänen, ACS Nano, 2013, 7, 11166 CrossRef CAS PubMed.
- F. Zhang, M. Funahashi and N. Tamaoki, Org. Electron., 2010, 11, 363 CrossRef CAS PubMed.
- W. Zhu, D. B. Farmer, K. A. Jenkins, B. Ek, S. Oida, X. Li, J. Bucchignano, S. Dawes, E. A. Duch and P. Avouris, Appl. Phys. Lett., 2013, 102, 233102 CrossRef PubMed.
- T. Yoon, W. C. Shin, T. Y. Kim, J. H. Mun, T.-S. Kim and B. J. Cho, Nano Lett., 2012, 12, 1448 CrossRef CAS PubMed.
- M. Imai, K. Akiyama, T. Tanaka and E. Sano, Compos. Sci. Technol., 2010, 70, 1564 CrossRef CAS PubMed.
- L. Jabbour, D. Chaussy, B. Eyraud and D. Beneventi, Compos. Sci. Technol., 2012, 72, 616 CrossRef CAS PubMed.
- B. Fugetsu, E. Sano, M. Sunada, Y. Sambongi, T. Shibuya, X. Wang and T. Hiraki, Carbon, 2008, 46, 1256 CrossRef CAS PubMed.
- M. Salajkova, L. Valentini, Q. Zhou and L. A. Berglund, Compos. Sci. Technol., 2013, 87, 103 CrossRef CAS PubMed.
- T. Oya and T. Ogino, Carbon, 2008, 46, 169 CrossRef CAS PubMed.
- R. E. Anderson, J. Guan, M. Ricard, G. Dubey, J. Su, G. Lopinski, G. Dorris, O. Bourne and B. Simard, J. Mater. Chem., 2010, 20, 2400 RSC.
- L. Hu, J. W. Choi, Y. Yang, S. Jeong, F. L. Mantia, L.-F. Cui and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21490 CrossRef CAS PubMed.
- S. H. Yoon, H.-J. Jin, M.-C. Kook and Y. R. Pyun, Biomacromolecules, 2006, 7, 1280 CrossRef CAS PubMed.
- M. Agarwal, Q. Xing, B. S. Shim, N. Kotov, K. Varahramyan and Y. Lvov, Nanotechnology, 2009, 20, 215602 CrossRef PubMed.
- L. Hu, M. Pasta, F. L. Mantia, L. Cui, S. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708 CrossRef CAS PubMed.
- J. G. Park, J. Smithyman, C.-Y. Lin, A. Cooke, A. W. Kismarahardja, S. Li, R. Liang, J. S. Brooks, C. Zhang and B. Wang, J. Appl. Phys., 2009, 106, 104310 CrossRef PubMed.
- L. Hu, H. Wu, F. L. Mantia, Y. Yang and Y. Cui, ACS Nano, 2010, 4, 5843 CrossRef CAS PubMed.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Phys. Rep., 2005, 409, 47 CrossRef PubMed.
- K. Ghule, A. V. Ghule, B.-J. Chen and Y.-C. Ling, Green Chem., 2006, 8, 1034 RSC.
| This journal is © The Royal Society of Chemistry 2015 |