Structure and hydrothermal stability of highly oriented polyamide 6 produced by solid hot stretching
Kaihua Shi,
Lin Ye* and
Guangxian Li
State Key Laboratory of Polymer Materials Engineering, Polymer Research Institute of Sichuan University, Chengdu, 610065, China. E-mail: yelinwh@126.com; Fax: +86-28-85402465; Tel: +86-28-85408802
First published on 23rd March 2015
Abstract
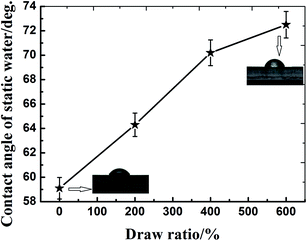
Highly oriented polyamide 6 (PA6) was successfully fabricated through solid hot stretching technology. The effect of orientation on the structure and hydrothermal stability of PA6 was investigated. It was found that molecular orientation reduced the hydrophilicity of PA6 and restricted the water uptake, and therefore hindered molecular hydrolytic degradation. The α crystalline form was mainly formed for the oriented PA6. With the increase of draw ratio, the crystallinity and orientation factor of PA6 increased, the fractional free volume (fv) decreased, and a dense crystalline structure was formed. During aging, the crystalline form of PA6 changed from γ to α, and thus compared with the isotropic sample, the oriented sample can retain a relatively stable dense crystalline structure, which was favorable for the inhibition of water diffusion into PA6 and slowing down the hydrolytic degradation. This investigation clearly showed that molecular orientation can be an efficient way to enhance the hydrothermal stability of PA6.
1 Introduction
Polyamide 6 (PA6), as one of the most common engineering plastics, has a wide range of applications in industry due to its excellent mechanical properties, oil resistance, and abrasive resistance.1,2 However, amide groups in the backbone of PA6 are sensitive to moisture in the environment, and undergo a reversible hydrolysis reaction, resulting in obvious deterioration of chemical structures and physical performances, and consequently limiting the use of polyamide materials.3–5 The degradation of polyamides when exposed to water has been examined by some scholars.5–7 A common aspect of these researches focused on degradation-dependent changes in mechanical properties, sample weight and water diffusion. N. S. Murthy et al.6 found that water molecules diffused almost exclusively into the amorphous regions of PA6. Diffusion of water into the interlamellar regions swelled this amorphous matrix, increased the lamellar repeat, decreased the unit cell volume in preexisting lamellae, and at elevated temperatures hydrolyzed the tie molecules. D. P. N. Vlasveld7 et al. studied the moisture absorption in PA6 silicate nanocomposites and its influence on the mechanical properties. They found that the nanocomposites absorbed water at a slower rate than the unfilled PA6 samples, and the modulus would eventually drop after enough moisture was absorbed. In a more recent study, Shu et al.5 studied the aging behavior of PA6 under acid rain in terms of water absorption behavior, mechanical property, chemical structure, and appearance properties. The stabilization of PA6 mostly focused on the thermal-oxidative and ultraviolet-oxidative stabilization,8–11 while few literatures on hydrothermal stabilization can be available for PA6.Polymer secondary structure such as chain crystalline and orientation has significant effect on its stability and aging behavior.12–14 Eldsäter C et al.12 found that selective degradation induced by extra cellular enzymes occurred in the amorphous part of oriented poly(ε-caprolactone) (PCL) films prior to the crystalline structure. Do Kwang Cho et al.13 studied the effect of molecular orientation on biodegradability of poly(glycolide-co-ε-caprolactone) (PGCL), and found that the degradation decreased with increasing draw ratio. Zhou Y et al.14 investigated the effect of crystallization on hydrolytic stability of polycarbonate (PC) and confirmed that solvent-induced crystallization on surface can be an efficient way to enhance the hydrolytic stability of PC.
In this work, highly oriented PA6 was successfully fabricated through solid hot stretching technology. The effect of orientation on the structure and hydrothermal stability of PA6 was investigated for the first time, and the enhancing mechanism of hydrothermal stability of PA6 was also explored.
2 Experimental
2.1 Materials
PA6 used in this work is a commercial grade granular product (YH800) without any additives and supplied by Yueyang Petrochemical Co. (Hunan, China), with relative viscosity of 2.85 ± 0.03. All the other solvents and reagents were used as-received.2.2 Preparation of the oriented PA6
 | (1) |
2.3 Hydrothermal aging
Pre-weighed samples of PA6 (W0) were immersed in separate beakers containing 500 mL deionized water at a constant temperature of 85 °C. At predetermined periods, the PA6 samples were picked out and wiped with filter paper to remove surface water. The wet weight (Ww) was measured immediately, and these specimens were then dried to a constant weight at 40 °C to weigh again (Wd).The water absorption and the weight gain of PA6 samples were obtained with the following equations.
 | (2) |
 | (3) |
2.4 Characterization
Static water contact angle measurements of the PA6 samples were conducted with DSA30 Krüss contact angle goniometer (KRÜSS GmbH, Germany) at 25 °C and 50% RH. Average values of contact angles were deduced from a total of at least ten measurements on different areas of each specimen.The intrinsic viscosities [η] of the PA6 samples were measured with an Ubbelohde viscometer at 25 °C in formic acid at approximately 0.5% polymer concentration through a single point method according to the following equation:
 | (4) |
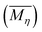 through the Mark–Houwink equation:
through the Mark–Houwink equation:
 | (5) |
The mechanical properties of PA6 samples were measured by Instron 5567 material testing machine (Instron Co., USA) according to ISO 527-1:2012(E). The test speed was 20 mm min−1, and the sample length between benchmarks was 20 mm.
Dynamic mechanical analysis (DMA) of PA6 samples was performed by using a TA Instrument Q800 DMA (TA Instruments, USA). Samples were analyzed over the temperature range of −100 °C to 150 °C at a heating rate of 3 °C min−1 and a frequency of 1 Hz in a strain mode. The sample size was 14 mm in length.
Microstructures of the PA6 samples were recorded with JSM-5900LV scanning electron microscope (SEM) (JEOL Ltd., Japan) with an acceleration voltage of 20 kV. Before SEM observation, the samples were sputter-coated with gold for 2–3 min.
The thermal properties of PA6 samples were performed with a Netzsch 204 differential scanning calorimetry (DSC) (Phoenix Co, Germany). The temperature scale of DSC was calibrated with indium. Granulated samples of about 10 mg were heated from ambient temperature to 250 °C at a constant rate of 10 K min−1 under nitrogen atmosphere. The crystallinity (Xc) can be calculated with the following equation:
| Xc = (ΔHm/ΔH0) × 100% | (6) |
Two-dimensional wide-angle X-ray diffraction (2D-WAXD) analysis was conducted at ambient temperature using a D8 Discover two-dimensional wide angle X-ray diffractometer (Bruker AXS Co, Germany). The sampling time of 2D-WAXD measurements was 180 s using an Eulerian 1/4 cradle HI-STAR (2D-Detector) detector, with a wavelength of 0.154 nm monochromated X-ray obtained from Cu (Kα) radiation.
The positron annihilation lifetime spectroscopy (PALS) of PA6 samples were measured by a conventional fast–fast coincidence spectrometer at room temperature. A 30 μCi 22 Na positron source sealed between two sheets of nickel foil (1 mg cm−2) was sandwiched between two pieces of the samples. About 106 data points were collected per spectrum. The third component (τ3 ≈ 1–3 ns) originated from ortho-positronium interacts with the surrounding electron densities and showed systematic variation with free volume. The lifetime and intensity of τ3 can be used to illustrate the size and concentration of free volume holes.
3 Results and discussion
3.1 Hydrothermal aging behavior of the oriented PA6
Theoretically, for the boundary condition, when the concentration of the solution on the surface of sample is constant and no chemical reaction occurs during the process of immersion, water absorption should be a Fick's process, which can be characterized by a constant coefficient of diffusion (D) and an equilibrium saturation water absorption (M∞), and obeying the following relationship:18
 | (7) |
In the initial process of immersion, the curve should have a good linear relationship as per the following formula:
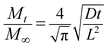 | (8) |
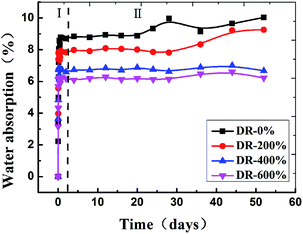
Then, water absorption (Mt) of PA6 was further plotted vs. t1/2/L (L being the sample thickness), as shown in Fig. 2. Eqn (8) was fitted to the experimental data using a linear regression analysis at a level of confidence of 95%. According to the slope of line, D could be calculated when M∞ was determined and summarized in Table 1. The value of D for the isotropic sample was much higher than that of the oriented sample and decreased with the draw ratio. The orientation of PA6 reduced the mobility of polymer chains, and restricted the water uptake and swelling at the early stage of the aging process. Thus the hydrolytic degradation maybe inhibited to a certain degree.19
| Draw ratio (%) | 0 | 200 | 400 | 600 |
|---|---|---|---|---|
| M∞ (%) | 8.75 | 7.84 | 6.72 | 6.12 |
| D (mm2 s−1) | 9.89 × 10−5 | 2.40 × 10−5 | 1.02 × 10−5 | 7.12 × 10−6 |
Fig. 3 shows the weight gain (%) as a function of hydrothermal aging time for all PA6 samples. At first, the weight of the samples increased as a result of the hydrolysis of the amide groups of PA6. The incorporation of 1 mol H2O to the polymer, i.e. 18 g, per hydrolysis event was expected. The isotropic sample showed the most rapid increase in weight, and reached maximum in about 3 hours. Afterwards, the weight of the isotropic sample and the sample with 200% draw ratio began to drop sharply, indicating that lots of low molecular weight compounds were extracted from the matrix and dissolved in water. However, the weight dropped very slowly for the samples with higher draw ratio, indicating that orientation can inhibit the hydrolytic degradation of PA6.
A model accounting for autocatalysis by the generated carboxylic acid end groups is described with the following rate equation:20
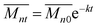 | (9) |
Based on the Mark–Houwink equation (eqn (5)), [η] is related to the viscosity average molecular weight,  , which is proportional to
, which is proportional to 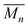 .21 Therefore, eqn (9) can be converted into an equation incorporating [η],
.21 Therefore, eqn (9) can be converted into an equation incorporating [η],
 | (10) |
| Draw ratio (%) | 0 | 200 | 400 | 600 |
|---|---|---|---|---|
| K1 × 103/day−1 | 10.04 | 8.94 | 6.36 | 3.98 |
| R2 | 0.97 | 0.97 | 0.97 | 0.96 |
The surface morphology of PA6 during the hydrothermal aging with varying draw ratio was shown in Fig. 8. It can be seen that the non-hydrolyzed undrawn PA6 samples showed a relatively smooth surface. As the draw ratio increased, the initial structure became more elongated along the principal tensile strain axis, and the micro-fibers structure was formed. Compared with the oriented samples, much more and wider cracks were observed for the isotropic sample during aging, while no obvious surface destruction occurred for the sample with DR-600%. Moreover, it's worth noting that more micro-fibers structure formed after hydrothermal aging for the oriented samples, suggesting that the erosion by hydrolysis occurred preferentially in the less ordered amorphous region between the oriented fibrils formed along the drawing direction.
 | ||
| Fig. 8 SEM images of PA6 with different draw ratio during the hydrothermal aging (magnification: 1000×). | ||
In this work, the cracks arranged parallel to the fiber texture at first and then perpendicular to the fiber texture. The amorphous region between lamellae became considerably weak and easily cracked along the lamellar direction by the selective degradation through the penetrating force of water into interlamellar region. The start of crystallization region degradation after complete hydrolysis of the amorphous regions perhaps led the cracks to arrange perpendicular to the fiber texture.
3.2 Hydrothermal stabilizing mechanism of the oriented PA6
 | ||
| Fig. 10 Crystalline morphology of etched surface of PA6 with different draw ratio (magnification: 200×). | ||
The DSC thermograms of isotropic and drawn samples of PA6 were shown in Fig. 11, from which the melting temperature (Tm), the heat of melting (ΔHm) and the crystallinity (Xc) can be obtained, as listed in Table 3.
| Draw ratio (%) | Tm (°C) | ΔH (J g−1) | Crystallinity (%) | Left area (%) | Right area (%) |
|---|---|---|---|---|---|
| 0 | 223.3 | 74.56 | 39.24 | 23.31 | 76.69 |
| 200 | 221.9 | 85.44 | 44.97 | — | — |
| 400 | 221.1 | 86.31 | 45.42 | — | — |
| 600 | 221.1 | 101.9 | 53.63 | — | — |
The melting endotherm of an isotropic PA6 sample exhibited a peak around 222 °C and an additional small shoulder peak at the lower temperature side of the melting peak was observed, and the cold crystalline was very obvious, indicating the imperfect crystallization of the sample. However, for the oriented sample, the shoulder peak and cold crystalline disappeared, and the melting temperature (Tm) decreased with the draw ratio, indicating the perfect crystallization of the sample, and the transformation of crystalline form γ to α because the crystalline form α with a more stable crystalline structure than crystalline form γ has a relatively low melting temperature.27 In addition, with the increase of draw ratio, the melting enthalpy and crystallinity of PA6 increased, as shown in Table 3, indicating of the stress-induced crystallization of PA6 during drawing.
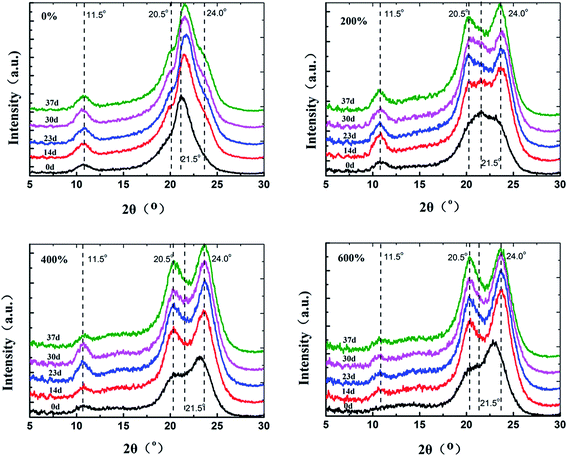
The 2D-XRD patterns of the PA6 during the hydrothermal aging with varying draw ratio were shown in Fig. 12. The isotropic sample before drawing presented a series of uniform Debye–Scherer diffraction rings due to the random arrangement of grains. While for the samples oriented, the (200) and (002)/(202) reflection appeared as two strong circular spots on the equator. With increasing draw ratio, these arcs became narrower in spread and more prominent, suggesting that the crystal axis was preferentially oriented perpendicular to the draw direction. With increase of aging time, the (200) and (002)/(202) reflection of PA6 became more prominent, suggesting that during aging the degree of the order of PA6 increased.
One-dimensional X-ray diffraction curve corresponding to the two-dimensional diffraction pattern was shown in Fig. 13. For isotropic PA6, the diffraction peak at 2θ = 21.5° was observed corresponding to the reflection of (200)/(001) of the γ crystal form. There were two new diffraction peaks for the oriented samples at 2θ = 20° and 23°, which could be assigned to the reflection of (200) and (002)/(202) of the α crystal form of PA6, respectively. In addition, the intensity of the diffraction peaks at 2θ = 23° increased with the draw ratio. The results indicated the transformation of γ crystalline form to α-form after drawn.
The crystallinity of PA6 can be calculated by the peak area of crystal and amorphous region form the corresponding decomposed curves obtained by PeakFit software. Azimuthal scans (0–360°) were performed for the characteristic reflections of PA6, and the Fit-2d package was employed to analyze the resultant 2D-WAXD patterns. The orientation factor (f) was calculated using Herman's orientation function:28–30
 | (11) |
 | (12) |
| Draw ratio (%) | Crystallinity (%) | Orientation factor | Grain size (Å) |
|---|---|---|---|
| 0 | 42.35 | — | 38 |
| 200 | 58.52 | 0.17 | 24 |
| 400 | 59.07 | 0.18 | 22 |
| 600 | 62.11 | 0.21 | 27 |
When the grain size is below 10 μm, the polycrystal X-ray diffraction peak broadens remarkably. Based on the widen amount of diffraction peak, the grain size can be calculated with the following equation:31
 | (13) |
As shown in Table 4, with the increase of the draw ratio, the crystallinity and oriented factor of PA6 increased, while the grain size of PA6 decreased, indicating that slipping and rupture of the lamellar in the spherulite occurred during the drawing of PA6 and a clear orientation of PA6 molecules formed.
Fig. 14 shows the corresponding one-dimensional X-ray diffraction curves of PA6 as a function of aging time. It can be seen that phase transition from γ-to α-crystals occurred during hydrothermal aging. In the presence of moisture, the chains in the meta-stable γ-phase are sufficiently mobile to assume the more stable, lower energy conformation, hence partially converting them to the α-form. Compared with the isotropic sample, the oriented samples could keep relatively stable dense crystalline structure due to its formed α-form by drawn.
The crystallinity, grain size and orientation factor calculated from the XRD scans as a function of aging time is shown in Fig. 15. The crystallinity and grain size of isotropic PA6 increased at the initial stage of aging, indicating the fact that post crystallization occurred as a result of the aging process. In addition, the hydrothermal degradation would primarily occur in the amorphous region of PA6, resulting in the increase of the crystalline region.32,33 After 30 days of aging, the crystallinity and grain size decreased, ascribing to the degradation of crystallization regions after the complete degradation of the amorphous regions. In comparison, for the oriented sample, the crystallinity, grain size and orientation factor changed slightly with time, indicating that the oriented sample could keep relatively stable crystal orientation structure. In this case, the orientation and crystalline domains played an important role in slowing down the hydrolysis.
 | ||
| Fig. 15 Grain size, crystallinity and orientation factor of PA6 as a function of hydrothermal aging time. | ||
The glass transition temperature (Tg) of PA6 samples was measured by DMA analysis, and the results was shown in Fig. 16. For the isotropic sample of PA6, in the initial stage of aging, lots of water diffused into the PA6 matrix, functioning as plasticizer and resulting in a decrease of Tg, and after 20 days, a slight increase of Tg can be observed due to the increase of crystallinity and degradation of the amorphous region. The Tg of PA6 moved to higher temperatures with increasing of draw ratio, which indicated that the local motions of molecular chains in the amorphous region and defect region within the crystal were suppressed, and moreover the Tg for the oriented samples kept relatively stable during hydrothermal aging.
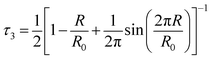 | (14) |
The free volume fraction can be estimated according to the following empirical formula:
| fv = AVhI3 | (15) |
As shown in Table 5, compared with the isotropic PA6, the hole radius (R) changed slightly, and the fractional free volume (fv) decreased for the oriented sample, resulting from the orientation of the amorphous region and the merger of the free volume hole. It can be seen that for the isotropic PA6, both fv and R showed a decrease for the aged sample, indicating that the small molecules and low molecular weight PA6 produced by degradation in the amorphous region were suitable to join the polymeric crystallites, while a slight change occurred for oriented PA6. The second lifetime peak (I2) of positrons was related with the crystalline/amorphous interfacial defects of the crystalline lamellae.36 After aging, the intensity of I2 for the isotropic PA6 increased, indicating the increase of the defects in crystalline region, while a slight change of I2 and a more stable structure were observed for the oriented sample of PA6.
| Time/days | Draw ratio (%) | I2 (%) | I3 (%) | τ3 (ps) | R (nm) | fv |
|---|---|---|---|---|---|---|
| 0 | 0 | 58.45 | 20.92 | 1.64 | 0.2500 | 2.463 × 10−3 |
| 400 | 61.13 | 19.17 | 1.65 | 0.2507 | 2.277 × 10−3 | |
| 42 | 0 | 60.40 | 19.61 | 1.61 | 0.2461 | 2.203 × 10−3 |
| 400 | 61.12 | 18.99 | 1.63 | 0.2481 | 2.185 × 10−3 |
4 Conclusion
Highly oriented PA6 was successfully fabricated through solid hot stretching technology. The effect of orientation on the structure and hydrothermal stability of PA6 was investigated. It was found that with the increase of draw ratio, the crystallinity and orientation factor of PA6 increased, and micro-fibre structure was formed, while the hydrophilic property was weakened. During hydrothermal aging, the equilibrium water absorption ratio and diffusion coefficient (D) of water decreased with the increase of draw ratio, and the oriented samples kept relatively higher reduced viscosity and mechanical property. The sample became yellowing and surface cracked during aging, which were remarkably reduced by orientation. The enhancing mechanism of hydrothermal stability of PA6 was explored, and the stable dense crystalline structure with α crystal form formed by orientation contributed to slowing down the hydrolytic degradation.Acknowledgements
This study was financially supported by the Key Natural Science Foundation of China (Grant no. 51133005).References
- L. Li and G. Yang, Variable-temperature FTIR studies on thermal stability of hydrogen bonding in nylon 6/mesoporous silica nanocomposite, Polym. Int., 2009, 58, 503–510 CrossRef CAS.
- Z. Hu, L. Chen, G.-P. Lin, Y. Luo and Y.-Z. Wang, Flame retardation of glass-fibre-reinforced polyamide 6 by a novel metal salt of alkylphosphinic acid, Polym. Degrad. Stab., 2011, 96, 1538–1545 CrossRef CAS PubMed.
- E. S. Gonçalves, L. Poulsen and P. R. Ogilby, Mechanism of the temperature-dependent degradation of polyamide 66 films exposed to water, Polym. Degrad. Stab., 2007, 92, 1977–1985 CrossRef PubMed.
- C. El-Mazry, O. Correc and X. Colin, A new kinetic model for predicting polyamide 6-6 hydrolysis and its mechanical embrittlement, Polym. Degrad. Stab., 2012, 97, 1049–1059 CrossRef CAS PubMed.
- Y. Shu, X. Li and L. Ye, Study on the Long-Term Acid Rain Aging Behaviour of Polyamide 6, J. Macromol. Sci., Part B: Phys., 2009, 48, 526–536 CrossRef CAS.
- N. S. Murthy, M. Stamm and J. P. Sibilia, et al., Structural changes accompanying hydration in nylon 6, Macromolecules, 1989, 22(3), 1261–1267 CrossRef CAS.
- D. P. N. Vlasveld, J. Groenewold and H. E. N. Bersee, et al., Moisture absorption in polyamide-6 silicate nanocomposites and its influence on the mechanical properties, Polymer, 2005, 46(26), 12567–12576 CrossRef CAS PubMed.
- T. Yang, L. Ye and Y. Shu, Thermal stabilization effect of biphenol monoacrylate on polyamide 6, J. Appl. Polym. Sci., 2008, 110(2), 856–863 CrossRef CAS.
- K. Shi and L. Ye, In situ stabilization of polyamide 6 with reactive antioxidant, J. Therm. Anal. Calorim., 2015, 119, 1747–1757 CrossRef CAS.
- D. Forsström and B. Terselius, Thermo oxidative stability of polyamide 6 films I. Mechanical and chemical characterisation, Polym. Degrad. Stab., 2000, 67(1), 69–78 CrossRef.
- K. Shi and L. Ye, Photo-Oxidative Stabilization Effect of a Reactive Hindered Amine on Monomer Casting Nylon-6, J. Macromol. Sci., Part B: Phys., 2014, 53(8), 1453–1464 CrossRef CAS.
- C. Eldsäter, B. Erlandsson, R. Renstad, A. C. Albertsson and S. Karlsson, The biodegradation of amorphous and crystalline regions in film-blown poly(ε-caprolactone), Polymer, 2000, 41, 1297–1304 CrossRef.
- D. K. Cho, J. W. Park, S. H. Kim, Y. H. Kim and S. S. Im, Effect of molecular orientation on biodegradability of poly(glycolide-co-ε-caprolactone), Polym. Degrad. Stab., 2003, 80, 223–232 CrossRef CAS.
- Y. Zhou, Y. Dan, L. Jiang and G. Li, The effect of crystallization on hydrolytic stability of polycarbonate, Polym. Degrad. Stab., 2013, 98, 1465–1472 CrossRef CAS PubMed.
- O. F. Solomon and I. Z. Ciutǎ, Détermination de la viscosité intrinsèque de solutions de polymères par une simple détermination de la viscosité, J. Appl. Polym. Sci., 1962, 6, 683–686 CrossRef CAS.
- I. Campoy, M. A. Gomez and C. Marco, Structure and thermal properties of blends of nylon 6 and a liquid crystal copolyester, Polymer, 1998, 39(25), 6279–6288 CrossRef CAS.
- J. S. Wiggins, M. K. Hassan and K. A. Mauritz, et al., Hydrolytic degradation of poly(D,L-lactide) as a function of end group: carboxylic acid vs. hydroxyl, Polymer, 2006, 47(6), 1960–1969 CrossRef CAS PubMed.
- W. Tham, Z. M. Ishak and W. Chow, Water Absorption and Hygrothermal Aging Behaviors of SEBS-g-MAH Toughened Poly(lactic acid)/Halloysite Nanocomposites, Polym.-Plast. Technol. Eng., 2014, 53, 472–480 CrossRef CAS.
- E. S. Gonçalves, L. Poulsen and P. R. Ogilby, Mechanism of the temperature-dependent degradation of polyamide 66 films exposed to water, Polym. Degrad. Stab., 2007, 92, 1977–1985 CrossRef PubMed.
- C. G. Pitt and G. Zhong-wei, Modification of the rates of chain cleavage of poly(ε-caprolactone) and related polyesters in the solid state, J. Controlled Release, 1987, 4, 283–292 CrossRef CAS.
- Q. Zhou and M. Xanthos, Nanoclay and crystallinity effects on the hydrolytic degradation of polylactides, Polym. Degrad. Stab., 2008, 93, 1450–1459 CrossRef CAS PubMed.
- R. Li and X. Hu, Study on discoloration mechanism of polyamide 6 during thermo-oxidative degradation, Polym. Degrad. Stab., 1998, 62, 523–528 CrossRef CAS.
- N. Zhao, L. Weng, X. Zhang, Q. Xie, X. Zhang and J. Xu, A Lotus-Leaf-Like Superhydrophobic Surface Prepared by Solvent-Induced Crystallization, ChemPhysChem, 2006, 7, 824–827 CrossRef CAS PubMed.
- R. N. Wenzel, Resistance of solid surfaces to wetting by water, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
- S. Herminghaus, Roughness-induced non-wetting, Europhys. Lett., 2000, 52, 165 CrossRef.
- X. Zhao and L. Ye, Structure and properties of highly oriented polyoxymethylene produced by hot stretching, Mater. Sci. Eng., A, 2011, 528(13), 4585–4591 CrossRef PubMed.
- D. Holmes, C. Bunn and D. Smith, The crystal structure of polycaproamide: nylon 6, J. Polym. Sci., 1955, 17, 159–177 CrossRef CAS.
- X. Zhang, F. Shi and J. Niu, et al., Superhydrophobic surfaces: from structural control to functional application, J. Mater. Chem., 2008, 18(6), 621–633 RSC.
- W. Rungswang, K. Plailahan and P. Saendee, et al., Tensile deformation of in-reactor polymer alloy with preferentially oriented crystallite in parallel and perpendicular to uniaxial stretching direction: a model case from impact-resistance polypropylene copolymer, Polymer, 2013, 54(14), 3699–3708 CrossRef CAS PubMed.
- Q. Liu, X. Sun and H. Li, et al., Orientation-induced crystallization of isotactic polypropylene, Polymer, 2013, 54(17), 4404–4421 CrossRef CAS PubMed.
- M. Fourati, C. Pellerin and C. G. Bazuin, et al., Infrared and fluorescence spectroscopy investigation of the orientation of two fluorophores in stretched polymer films, Polymer, 2013, 54(2), 730–736 CrossRef CAS PubMed.
- Y.-B. Luo, X.-L. Wang and Y.-Z. Wang, Effect of TiO2 nanoparticles on the long-term hydrolytic degradation behavior of PLA, Polym. Degrad. Stab., 2012, 97, 721–728 CrossRef CAS PubMed.
- D. Rangari and N. Vasanthan, Study of Strain-Induced Crystallization and Enzymatic Degradation of Drawn Poly(L-lactic acid) (PLLA) Films, Macromolecules, 2012, 45, 7397–7403 CrossRef CAS.
- M. Eldrup, D. Lightbody and J. N. Sherwood, The temperature dependence of positron lifetimes in solid pivalic acid, Chem. Phys., 1981, 63, 51–58 CrossRef CAS.
- Y. Y. Wang, H. Nakanishi, Y. C. Jean and T. C. Sandreczki, Positron annihilation in amine-cured epoxy polymers—pressure dependence, J. Polym. Sci., Part B: Polym. Phys., 1990, 28, 1431–1441 CrossRef CAS.
- M. A. Monge, J. A. Díaz and R. Pareja, Strain-Induced Changes of Free Volume Measured by Positron Lifetime Spectroscopy in Ultrahigh Molecular Weight Polyethylene, Macromolecules, 2004, 37, 7223–7230 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |