DOI:
10.1039/C5RA01968K
(Paper)
RSC Adv., 2015,
5, 37823-37829
Tetraamino-zinc phthalocyanine covalently bound to benzoic acid-functionalized graphene composites for highly efficient visible light photocatalytic activities
Received
6th February 2015
, Accepted 20th April 2015
First published on 20th April 2015
Abstract
A composite of 1,8,15,22-tetraamino zinc phthalocyanine (ZnTAPc) covalently bound to benzoic acid-functionalized graphene (BFG) was used for photodegradation of rhodamine B (RhB) for the first time. The formation of an amido bond between ZnTAPc and BFG has been confirmed by Fourier transform infrared spectroscopy, Raman spectroscopy and X-ray photoelectron spectroscopy. For photodegradation of RhB in water, the as-prepared ZnTAPc–10% BFG composite exhibits much stronger visible-light catalytic activity than pure ZnTAPc. This effect can be attributed to the high pollutant adsorption performance of ZnTAPc–BFG, the increased light absorption and the effective charge transfer and separation. In addition, the excess BFG (15%) in the composite results in a significant decrease in photocatalytic efficiency, due to the decrease of the adsorption sites and catalytic active centers. The ZnTAPc–10% BFG composite photocatalyst, with high photocatalytic efficiency and reusability, is potentially applicable in environmental purification of organic pollutants.
1. Introduction
In the past decades, there has been great interest in developing photocatalysts with high catalytic efficiency and good stability for water splitting and removal of hazardous organic compounds in industrial wastewater.1–3 As a class of typical conjugated organic molecules, porphyrins have often been used as attractive building blocks for functional structures because of their tailored electronic, optical and catalytic properties via rational molecular design.4–6 To date, some metal phthalocyanines (MPcs) and their derivatives, based on porphyrin building blocks, have been developed for degradation of organic pollutants in water under sunlight7–9 since they have intense absorption bands in the longer wavelength region of the visible spectrum. However, the photocatalytic efficiencies of pure MPcs are far from efficient for practical applications under visible light. Therefore, it is necessary to find an effective way to change this situation.
Functionalized graphenes with a 2D single atomic layer structure, especially reduced graphene oxide (RGO), have attracted increasing interests both in fundamental science and for wide potential applications, due to its excellent electronic properties, superior chemical stability and high specific surface area. For example, Geng and co-workers demonstrated a photoinduced electron transfer from porphyrins to RGO in the multiple-bilayered RGO–porphyrin film.10 Loh et al. found that the addition of pyridine-functionalized RGO can change the crystallization process of iron–porphyrin in the MOF, increase its porosity and enhance the electrochemical charge transfer rate of iron–porphyrin.11 Huang's group reported that porphyrin/RGO nanohybrid in the form of free-standing film exhibits enhanced visible-light photocatalytic activity for degradation of organic pollutants compared to each moiety of the hybrid.12
Benzoic acid-functionalized graphene (BFG) was synthesized by grafting the group of benzoic acid onto RGO via the aryl diazonium salt reaction, which can act as both a structure-directing template and a framework linker to produce interesting structural motif.13,14 Recently, a composite of tetraamino-zinc phthalocyanine (ZnTAPc) covalently bound to benzoic acid-functionalized graphene (BFG) has been reported to exhibit stronger nonlinear optical properties compared to ZnTAPc.15 To the best of our knowledge, there have been no reports on the modification of ZnTAPc with BFG for the purpose of photodegradation of organic pollutants. It can be expected that the combination of ZnTAPc with BFG may improve the photogenerated electron transfer and separation during the photodegradation reactions.
In the present work, the composites of ZnTAPc covalently bound to BFG were used for photodegradation of rhodamine B (RhB) under visible light irradiation (λ > 400 nm) for the first time. The causes for the increased photocatalytic activity of the composites were studied by means of various characterization methods. The results of this work reveal that ZnTAPc–10% BFG composite exhibits much higher photocatalytic activity than pure ZnTAPc for degradation of RhB. The mechanism of photodegradation reaction catalyzed by ZnTAPc–BFG is discussed.
2. Experimental section
2.1 Apparatus and reagents
All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd and used without further purification. Transmission electron microscopy (TEM) analysis was performed on a JEM-2100F electron microscope. Fourier transform infrared spectra (FTIR) of the samples were collected on a Nicolet 67 spectrometer using the KBr pellet technique. Raman spectra were recorded on a LabRAM HR Evolution spectrometer with an excitation of 532 nm laser light. The X-ray photoelectron spectroscopy (XPS) measurement was carried out using an EscaLab 250Xi spectrometer fitted with a monochromatic Al Kα X-ray source (hν = 1486.6 eV). UV-vis spectra of RhB solutions were obtained at room temperature with a Shimadzu UV-2550 spectrophotometer. UV-vis diffuse reflection spectroscopy of ZnTAPc and the composite was measured on a DUV-3700 spectrophotometer. Specific surface areas were calculated using the Brunauer–Emmett–Teller (BET) model via Quantachrome NOVA2200e nitrogen adsorption apparatus.
2.2 Preparation of ZnTAPc–BFG
As previously reported, BFG was synthesized by sonicating 300 mg RGO dispersed in 1 wt% aqueous sodium dodecylbenzenesulfonate (SDBS) surfactant,13 and 1,8,15,22-tetraamino-zinc phthalocyanine (ZnTAPc) was prepared from 3-nitro-phthalic anhydride, urea, ammonium molybdate and zinc acetate.16 The ZnTAPc–BFG composites were synthesized by amidation reaction (Scheme 1) on the basis of the literature15 with the improvement in acylation reaction using acyl chloride instead of BFG itself, for the purpose of adjusting the structure of the composites and increasing the catalytic active sites. BFG was firstly dissolved and refluxed in SOCl2 (20 mL) in the presence of DMF (0.5 mL) at 70 °C for 24 h under nitrogen atmosphere. The excess SOCl2 and solvent were removed by distillation. In the presence of triethylamine (Et3N, 0.5 mL), the above acyl chloride was allowed to react with ZnTAPc (30 mg) in DMF (15 mL) at 130 °C for 72 h under nitrogen. After the reaction, the solution was cooled to room temperature and then poured into ether (200 mL) to precipitate the product. The product was isolated by filtration on a microporous membrane of polyvinylidene fluoride (0.22 mm). The excess impurities were removed though sonication and re-suspension of the solid in tetrahydrofuran (50 mL). Finally the resulting suspensions were dried under vacuum to yield the ZnTAPc–BFG composites. By changing the amount of BFG added, the as-synthesized ZnTAPc–BFG composites with 5, 10 and 15 wt% BFG were named as ZnTAPc–5% BFG, ZnTAPc–10% BFG and ZnTAPc–15% BFG, respectively.
 |
| | Scheme 1 Illustration of formation of ZnTAPc–BFG. | |
2.3 Photocatalytic experiments
The photocatalytic degradation of RhB was measured at ambient pressure and 298 K in a set of home-made photochemical reaction equipment. The light source was a 300 W xenon lamp equipped with a cut-off filter (λ > 400 nm). The photocatalyst 5 mg was added into 10 mL RhB (10 mg L−1) aqueous solution. Before irradiation, the suspension was stirred continuously for 10.5 h in the dark in order to reach an adsorption–desorption equilibrium of RhB between the solution phase and the surface of the photocatalyst. The RhB concentration in the solution was examined by spectrophotometry after filtration by 0.22 μm filter.
3. Results and discussion
3.1 Transmission electron microscopy (TEM) analysis
The morphology of the as-prepared ZnTAPc and composites was examined by TEM. As expected, BFG shows transparent folded nanosheet-like morphology resembling crumpled silk veil waves with size of few hundred square nanometers (Fig. 1a). ZnTAPc consists of fish-scale nanoflakes with average diameters of 40–50 nm (Fig. 1b). With the addition of BFG, there is a change in the structure and morphology of the composites. For ZnTAPc–5% BFG composite, the irregular BFG nanosheets are attached with fish-scale ZnTAPc nanoflakes (Fig. 1c). In the case of 10% BFG, the composite shows a regular multi-laminated nanostructure with uniform size of about 20 nm (Fig. 1d), due to the bonding of ZnTAPc with the BFG nanosheets. This structure favors for the π–π stacking interaction and electron transport between ZnTAPc and BFG in the photocatalytic oxidation reaction. However, for ZnTAPc–15% BFG composite, few ZnTAPc nanoflakes can be distinguished due to the presence of excess BFG nanosheets (Fig. 1e), resulting in a decrease in photocatalytic active sites compared to ZnTAPc–10% BFG. By comparison, ZnTAPc–10% BFG composite has the most uniform morphology and is expected to have the most active sites of photodegradation reaction among all the tested samples. HRTEM was used to further differentiate the ZnTAPc phase from the BFG phase, as this technique gives a resolution at the atomic scale. The obtained HRTEM image (Fig. 1f) reveals the crystal lattice planes assigned to the crystalline ZnTAPc and the amorphous phase attributed to BFG.
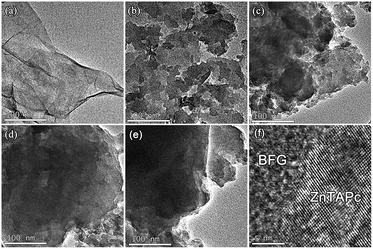 |
| | Fig. 1 TEM images of BFG (a), ZnTAPc (b), ZnTAPc–5% BFG (c), ZnTAPc–10% BFG (d), ZnTAPc–15% BFG (e) and HRTEM image of ZnTAPc–BFG (f). | |
3.2 FT-IR analysis
Fig. 2 shows FTIR spectra of BFG, ZnTAPc and ZnTAPc–10% BFG composite. In the case of BFG, the peak at 1700 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O characteristic stretching of the free carboxylic group. While in the case of ZnTAPc–10% BFG composite, two new bands emerge at 1636 and 1260 cm−1 corresponding to the stretching bands of C
O characteristic stretching of the free carboxylic group. While in the case of ZnTAPc–10% BFG composite, two new bands emerge at 1636 and 1260 cm−1 corresponding to the stretching bands of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N from the amide group,17 respectively. These results clearly indicate that the ZnTAPc molecules are covalently bound to the BFG by the amide linkage.
O and C–N from the amide group,17 respectively. These results clearly indicate that the ZnTAPc molecules are covalently bound to the BFG by the amide linkage.
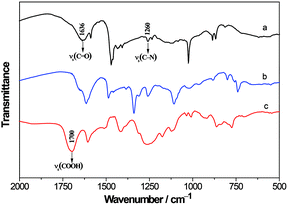 |
| | Fig. 2 FTIR spectra of ZnTAPc–10% BFG (a), ZnTAPc (b) and BFG (c). | |
3.3 Raman analysis
The significant π-electron interactions between ZnTAPc and the BFG basal plane were further confirmed by Raman spectroscopy. As shown in Fig. 3, a single peak at 747 cm−1 and a group of peaks in the range of 1300–1600 cm−1 are found for ZnTAPc. Two bands (D and G bands) are observed for both BFG and the composite. The D band (1300–1400 cm−1) is ascribed to disordered sp3 bonded carbons, vacancies or edges, whereas the G band (1500–1700 cm−1) is assigned to in-plane stretching of ordered sp2 bonded carbon. In the case of BFG, D band is at 1349 cm−1 and G band is at 1592 cm−1. For the composite, both D and G bands are found to be shifted and broadened due to peak overlapping between ZnTAPc and BFG in the composite, besides a very weak peak at 747 cm−1 coming from ZnTAPc.18 The D/G intensity ratio of 1.04 in the composite is higher than that of 0.91 in BFG, which can be explained by the introduction of sp3 defects during covalent functionalization of ZnTAPc with BFG.19
 |
| | Fig. 3 Raman spectra of ZnTAPc–10% BFG (a), ZnTAPc (b) and BFG (c). | |
3.4 XPS analysis
As shown in Fig. 4, the X-ray photoelectron spectra provide essential and useful information for the covalent attachment of the ZnTAPc moieties onto the surface of BFG. The C 1s spectrum of BFG exhibits a weak peak around 289.1 eV (COOH) and a strong multiple peak which can be decomposed two components at 284.1 eV (C–C) and 285.3 eV (C–N).20,21 After ZnTAPc moieties were loaded onto the surface of BFG, a new component peak at 287.3 eV was observed22 while the component peak at 289.1 eV disappeared (Fig. 3b), suggesting the formation of amido bond. The N 1s XPS spectrum of ZnTAPc can be decomposed to three components at 398.8 eV (N–C), 399.3 (N–H) and 400.1 eV (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C),23 respectively. For ZnTAPc–10% BFG, one component of N–C band shifted to lower binding energy by 0.9 eV while one component of N
C),23 respectively. For ZnTAPc–10% BFG, one component of N–C band shifted to lower binding energy by 0.9 eV while one component of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band shifted to higher binding energy by 0.8 eV, which indicate the charges transfer from ZnTAPc to BFG.
C band shifted to higher binding energy by 0.8 eV, which indicate the charges transfer from ZnTAPc to BFG.
 |
| | Fig. 4 X-ray photoelectron spectra for C 1s of BFG (a), ZnTAPc–10% BFG (b) and N 1s of ZnTAPc (c), ZnTAPc–10% BFG (d). | |
3.5 Adsorption activity of ZnTAPc–BFG composites
Fig. 5a shows a great advantage of the ZnTAPc–BFG composites over pure ZnTAPc in adsorbing the organic dye RhB from the solution, which may be attributed to the adsorption performance of the BFG nanosheets with a huge specific surface area and a large number of active groups. The kinetic data of adsorption processes were further analyzed (Fig. 5b) using a pseudo-second-order kinetics model proposed by Ho,24 which is based on the assumption the sorption follows second order chemisorption. The pseudo-second-order equation is given below:
where qe (mg g−1) and qt (mg g−1) are the adsorbed solute amount by adsorbent at equilibrium state and that at a time of t (min), respectively. The modeled results are listed in Table 1. The correlation coefficient values indicate a better fit of the pseudo-second-order equation with the experimental data compared to other kinetic models for all the composites. The values of qe calculated from the pseudo-second-order equation were 1.045, 4.134, 4.793 and 1.422 mg g−1 for ZnTAPc, ZnTAPc–5% BFG, ZnTAPc–10% BFG and ZnTAPc–15% BFG, respectively, indicating a chemisorption process.25
 |
| | Fig. 5 (a) Adsorption curves of RhB on ZnTAPc and ZnTAPc–BFG composite. (b) The pseudo-second-order kinetic of RhB adsorption for ZnTAPc and ZnTAPc–BFG composite. | |
Table 1 Constants and correlation coefficients for the kinetic models
| Sample |
qe (mg g−1) |
R2 |
| ZnTAPc |
1.045 |
0.9986 |
| ZnTAPc–5% BFG |
4.134 |
0.9979 |
| ZnTAPc–10% BFG |
4.793 |
0.9983 |
| ZnTAPc–15% BFG |
1.422 |
0.9999 |
The Brunauer–Emmett–Teller (BET) surface areas of ZnTAPc, ZnTAPc–5% BFG, ZnTAPc–10% BFG and ZnTAPc–15% BFG were measured for better understanding of the adsorption activity of ZnTAPc–BFG composites. The specific surface area of pure ZnTAPc is 1.75 m2 g−1, whereas the value of the composites with 5 and 10% BFG doping ratio increases to 6.56 and 8.08 m2 g−1, respectively. It is clear that the incorporation of BFG can increase the specific surface area of the composites so as to enhance the adsorption of organic dye. With further increase of BFG to 15%, the specific surface area decreases to 3.49 m2 g−1. It is thus presumed that the lower amount of ZnTAPc between adjacent BFG layers could not effectively inhibit the aggregation of BFG, resulting in much lower specific surface area. The lower specific surface area of ZnTAPc–15% BFG is considered as one of the main causes for the lower adsorptivity than the composites with 5% and 10% BFG.
3.6 Photocatalytic activity of ZnTAPc–BFG composites
RhB with a major absorption band at 553 nm was chosen as a representative hazardous organic dye to evaluate the photocatalytic activity of the samples under visible light. As shown in Fig. 6a, ZnTAPc shows a certain visible light photocatalytic activity. For the composites with 5% and 10% BFG, the visible light photocatalytic activity increases significantly with the addition of BFG. This may be due to the fact that the adsorption capacity of the composites for RhB is increased with the increase of BFG in the composites. However, the photocatalytic activity of the composite with 15% BFG decreases below that of pure ZnTAPc, at least partly due to the decrease of the adsorption sites and catalytic active centers.
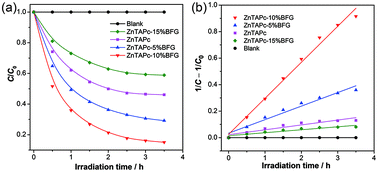 |
| | Fig. 6 (a) Photodegradation of RhB on ZnTAPc, the composites and blank. (b) The pseudo-second-order kinetics of RhB photodegradation for ZnTAPc, the composites and blank. | |
The kinetic data of the photodegradation reactions were linearized using the second order kinetic equation, and a satisfactory fit was obtained for all cases as shown in Fig. 6b. The apparent rate constant (k) and half-life (t1/2)26 of all the samples are then calculated and summarized in Table 2, from which we can see that the reaction rate constant increases in the following order: ZnTAPc–15% BFG < ZnTAPc < ZnTAPc–5% BFG < ZnTAPc–10% BFG. The ZnTAPc–10% BFG exhibits the fastest kinetics whereas ZnTAPc–15% BFG displays the slowest kinetics. The photocatalytic efficiency of ZnTAPc–10% BFG composite is higher than that of other graphene-based composites such as RGO–TiO2 and GO–g-C3N4 (Table 3).
Table 2 Photocatalytic degradation kinetic values for RhB
| Sample |
k × 102 (L mg−1 h−1) |
t1/2 (h) |
| ZnTAPc |
3.676 |
2.72 |
| ZnTAPc–5% BFG |
10.22 |
0.98 |
| ZnTAPc–10% BFG |
27.18 |
0.37 |
| ZnTAPc–15% BFG |
2.236 |
4.47 |
Table 3 Graphene-based composites for photodegradation of RhB
| Photocatalyst |
Time |
Degradation degree |
Reference |
| ZnTAPc–10% BFG |
2.5 h |
82% |
This work |
| RGO–TiO2 |
3 h |
67% |
27 |
| GO–g-C3N4 |
2.5 h |
68.1% |
28 |
It has been known that the excess BFG in ZnTAPc–15% BFG results in a decrease of specific surface area of the composite and thus adsorption capacity to RhB. A high adsorption capacity is a pre-requisite to attain a high photocatalytic activity,29 but it seems not to be the only reason in the present system. This conclusion is supported by the comparison of the photocatalytic rate constant with the adsorption capacity for all the samples (Fig. 7). Further discussion will be made on the basis of UV-vis absorption measurements in the next section.
 |
| | Fig. 7 The photocatalytic rate constant and adsorption capacity of ZnTAPc and the composites. | |
The regeneration of the photocatalyst is one of the important steps for practical applications. The recycle experiments of RhB degradation on the ZnTAPc–10% BFG sample were performed to further investigate the photocatalytic stability of the ZnTAPc–10% BFG (Fig. 8). After four cycles the photocatalytic activity remained almost as high as that in the first cycle, indicating that the composite photocatalyst is relatively stable during the photocatalytic degradation of RhB.
 |
| | Fig. 8 Photodegradation of RhB on the recycled ZnTAPc–10% BFG. | |
3.7 Photodegradation mechanism of RhB
To investigate the possible reaction mechanism of the decolorization of RhB under visible light irradiation over ZnTAPc–10% BFG, the generation of the primary active species including superoxide radical (O2˙−) and hydroxyl radical (HO˙) in the photodegradation process was explored (Fig. 9). Different scavengers, namely benzoquinone (BQ) for O2˙− and iso-propanol (IPA) for HO˙, were employed in this study, by adding the radical scavengers (1 mM) to the reaction system.30 The addition of IPA exhibited little influence on the dye generation compared to the ZnTAPc–10% BFG, suggesting that HO˙ minimally involved in the catalytic oxidation reaction. However, when benzoquinone was added into the reaction solution, the generation of dye was significantly inhibited. Therefore, the generation of O2˙− was crucial for the decolorization reaction and an O2˙− mediated mechanism of ZnTAPc catalysis is confirmed.31
 |
| | Fig. 9 Photodegradation of RhB on ZnTAPc–10% BFG with superoxide radical scavenger (BQ) and hydroxyl radical scavenger (IPA). | |
Fig. 10 shows the UV-vis diffuse reflectance spectra (DRS) of ZnTAPc and ZnTAPc–10% BFG composite. The composite showed stronger light absorption intensity in both UV and visible light regions than pure ZnTAPc. Furthermore, an obvious red shift to higher wavelength is observed in the absorption edge of the ZnTAPc–10% BFG composite. Therefore, the introduction of BFG is able to effectively promote the visible light response of the ZnTAPc–10% BFG composite, which can be attributed to electronic interactions between BFG and ZnTAPc. Such an extended optical absorption has also been observed in previous research works regarding graphene-semiconductor photocatalysts.32,33 The band-gap of the composites was calculated by using the equation of αhv = A(hv − Eg)n/2, where α, v, Eg and A are respectively absorption coefficient, light frequency, band-gap and a constant, and n depends on whether the transition is direct (n = 1) or indirect (n = 4).34 For ZnTAPc and ZnTAPc–10% BFG, the value of n is 1. A band-gap of 1.15 eV is obtained for ZnTAPc from the intercept of the tangents to the plots, whereas the band-gap of ZnTAPc–10% BFG is slightly reduced to 0.99 eV. Thus, the introduction of BFG to ZnTAPc causes the decrease in the bandgap, which is beneficial to the photocatalytic performance. The BFG bound to ZnTAPc can improve the transfer of photogenerated electrons and thus promote the separation of electron–hole pairs under irradiation. On the other hand, the excess BFG nanosheets in ZnTAPc–15% BFG not only obstruct the light absorption of ZnTAPc, but also prevent ZnTAPc from reaching the reactants in solution.
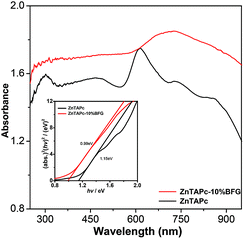 |
| | Fig. 10 Diffuse reflectance spectra of ZnTAPc and ZnTAPc–10% BFG. | |
The band structure of ZnTAPc sample was examined by valence band (VB) XPS as shown in Fig. 11. With VB XPS, the VB maxima of ZnTAPc is revealed to be 0.61 eV. The conduction band potential (VCB) of ZnTAPc was about −0.54 eV, which was negative than the standard redox potential of O2/O2˙− (−0.33 eV).35
 |
| | Fig. 11 VB XPS spectra of ZnTAPc. | |
On the basis of VB and CB levels of ZnTAPc, the photoelectron transfer mechanism for the decolorization of RhB over ZnTAPc–BFG composite under visible light might proceed through the following consecutive reactions:
| |
 | (1) |
| | |
3ZnTAPc* + O2 → ZnTAPc + O2˙−
| (2) |
| | |
3ZnTAPc* + RhB → ZnTAPc˙− + RhB˙+
| (3) |
| | |
ZnTAPc˙− + O2 → ZnTAPc + O2˙−
| (4) |
| | |
HO2˙ + RhB–H → H2O2 + RhB˙
| (6) |
| | |
RhB˙, RhB˙+ + HO˙ → oxidation products
| (8) |
(ISC: intersystem crossing).
When ZnTAPc–BFG composite was irradiated with visible light, the ZnTAPc molecule got excited to the singlet state (1ZnTAPc*), then underwent intersystem crossing transition to the triplet state (3ZnTAPc*). The 3ZnTAPc* species might produce radicals on interaction with the ground state molecular oxygen (eqn (2)) and the substrate molecule generating radical ions (eqn (3)). Then the O2˙− radical anions (eqn (4)) and HO˙ radicals (eqn (5)–(7)) were further formed, and the latter subsequently afforded oxidation of the substrate.
As shown in Fig. 12, electrons are excited from VB to CB of ZnTAPc under irradiation, leaving positive holes in the VB. Because of the perfect conductivity of graphene and the interfacial equilibrium of energy levels, the transfer of electrons from ZnTAPc to BFG is theoretically favorable. As a combiner of electron acceptor and transporter, BFG can efficiently inhibit the recombination of charge carriers. Therefore, the electrons transferred to BFG can be scavenged by the dissolved O2 to form O2˙− radical anions. Hydroxyl radicals (HO˙) are also formed because of the reaction between positively charged holes and H2O or OH−. The active species of hydroxyl radicals and superoxide radical anions can cause mineralization and oxidize organic pollutants into CO2 and H2O.
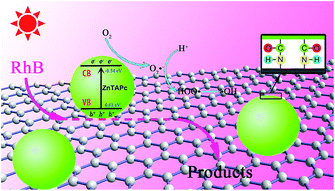 |
| | Fig. 12 Photocatalytic mechanism of ZnTAPc–BFG composite. | |
4. Conclusions
In conclusion, a composite of ZnTAPc covalently bound to BFG was synthesized by an amidation reaction, which was used for photodegradation of RhB for the first time. TEM results show that there is a change in terms of the structure and morphology of the composites with the addition of BFG, which can provide a greater number of active adsorption sites to improve the photocatalytic activity. The formation of an amido bond between ZnTAPc and BFG has been confirmed by Fourier transform infrared spectroscopy, Raman spectroscopy and X-ray photoelectron spectroscopy. For photodegradation of RhB in water under visible light, ZnTAPc–10% BFG composite exhibits much higher photocatalytic efficiency than pure ZnTAPc, which can be attributed to the high pollutant adsorption performance of ZnTAPc–BFG, the increased light absorption and the effective charge transfer and separation. However, the excess BFG (15%) in the composite results in a significantly decrease in photocatalytic efficiency, due to the decrease of the adsorption sites and catalytic active centers. With the high photocatalytic efficiency and reusability, the ZnTAPc–10% BFG composite photocatalyst is potentially applicable in environmental purification of organic pollutants. This work not only provides an example of a BFG-based composite photocatalyst and demonstrates that BFG is a very promising candidate for development of high performance photocatalysts, but also opens new possibilities to provide some insight into the design of high performance modified photocatalysts and other applications.
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (no. 51203040), Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20130111110025), Natural Science Foundation of Anhui Province (no. 1208085QB45) and Fundamental Research Funds for the Central Universities (no. 2014HGQC0016).
Notes and references
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- C. T. Dinh, H. Yen, F. Kleitz and T. O. Do, Angew. Chem., Int. Ed., 2014, 53, 6618–6623 CrossRef CAS PubMed.
- G. Z. Liao, S. Chen, X. Quan, H. T. Yu and H. M. Zhao, J. Mater. Chem., 2012, 22, 2721–2726 RSC.
- J. Rawson, A. C. Stuart, W. You and M. J. Therien, J. Am. Chem. Soc., 2014, 136, 17561–17569 CrossRef CAS PubMed.
- K. Mase, K. Ohkubo and S. Fukuzumi, Inorg. Chem., 2015, 54, 1808–1815 CrossRef CAS PubMed.
- M. A. Khaderbad, V. Tjoa, T. Z. Oo, J. Wei, M. Sheri, R. Mangalampalli, V. R. Rao, S. G. Mhaisalkar and N. Mathews, RSC Adv., 2012, 2, 4120–4124 RSC.
- G. Bottari, O. Trukhina, M. Ince and T. Torres, Coord. Chem. Rev., 2012, 256, 2453–2477 CrossRef CAS PubMed.
- D. Drozd, K. Szczubiałka, Ł. Łapok, M. Skiba, H. Patel, S. M. Gorun and M. Nowakowska, Appl. Catal., B, 2012, 125, 35–40 CrossRef CAS PubMed.
- Z. H. Zhang, M. J. Zhang, J. Deng, K. J. Deng, B. G. Zhang, K. L. Lv, J. Sun and L. Q. Chen, Appl. Catal., B, 2013, 132–133, 90–97 CrossRef CAS PubMed.
- J. H. Sun, D. L. Meng, S. D. Jiang, G. F. Wu, S. K. Yan, J. X. Geng and Y. Huang, J. Mater. Chem., 2012, 22, 18879–18886 RSC.
- M. Jahan, Q. L. Bao and K. P. Loh, J. Am. Chem. Soc., 2012, 134, 6707–6713 CrossRef CAS PubMed.
- Y. Z. Chen, Z. H. Huang, M. B. Yue and F. Y. Kang, Nanoscale, 2014, 6, 978–985 RSC.
- M. Jahan, Q. L. Bao, J. X. Yang and K. P. Loh, J. Am. Chem. Soc., 2010, 132, 14487–14495 CrossRef CAS PubMed.
- R. Kumar, K. Jayaramulu, T. K. Maji and C. N. R. Rao, Dalton Trans., 2014, 43, 7383–7386 RSC.
- C. Peng, Y. Z. Xiong, Z. B. Liu, F. Zhang, E. C. Ou, J. T. Qian, Y. Q. Xiong and W. J. Xu, Appl. Surf. Sci., 2013, 280, 914–919 CrossRef CAS PubMed.
- F. D. Cong, B. Ning, X. G. Du, C. Y. Ma, H. F. Yu and B. Chen, Dyes Pigm., 2005, 66, 149–154 CrossRef CAS PubMed.
- Z. B. Liu, Y. F. Xu, X. Y. Zhang, X. L. Zhang, Y. S. Chen and J. G. Tian, J. Phys. Chem. B, 2009, 113, 9681–9686 CrossRef CAS PubMed.
- J. P. Zou, J. Ma, Q. Huang, S. L. Luo, J. Yu, X. B. Luo, W. L. Dai, J. Sun, G. C. Guo, C. T. Au and S. L. Suib, Appl. Catal., B, 2014, 156–157, 447–455 CrossRef CAS PubMed.
- A. J. Wang, L. L. Long, W. Zhao, Y. L. Song, M. G. Humphrey, M. P. Cifuentes, X. Z. Wu, Y. S. Fu, D. D. Zhang, X. F. Li and C. Zhang, Carbon, 2013, 53, 327–338 CrossRef CAS PubMed.
- R. L. Arechederra, K. Artyushkova, P. Atanassov and S. D. Minteer, ACS Appl. Mater. Interfaces, 2010, 2, 3295–3302 CAS.
- H. K. He and C. Gao, Chem. Mater., 2010, 22, 5054–5064 CrossRef CAS.
- L. Cen, K. G. Neoh and E. T. Kang, Biosens. Bioelectron., 2003, 18, 363–374 CrossRef CAS.
- L. L. Cui, Y. Liu and X. Q. He, J. Electroanal. Chem., 2014, 727, 91–98 CrossRef CAS PubMed.
- Y. S. Ho, J. Hazard. Mater., 2006, 136, 681–689 CrossRef CAS PubMed.
- G. Crini, H. N. Peindy, F. Gimbert and C. Robert, Sep. Purif. Technol., 2007, 53, 97–110 CrossRef CAS PubMed.
- P. Xiong, L. J. Wang, X. Q. Sun, B. H. Xu and X. Wang, Ind. Eng. Chem. Res., 2013, 52, 10105–10113 CrossRef CAS.
- J. T. Zhang, Z. G. Xiong and X. S. Zhao, J. Mater. Chem., 2011, 21, 3634–3640 RSC.
- S. W. Hu, L. W. Yang, Y. Tian, X. L. Wei, J. W. Ding, J. X. Zhong and P. K. Chu, J. Colloid Interface Sci., 2014, 431, 42–49 CrossRef CAS PubMed.
- Q. Chen, Q. Q. He, M. M. Lv, X. T. Liu, J. Wang and J. P. Lv, Appl. Surf. Sci., 2014, 311, 230–238 CrossRef CAS PubMed.
- Y. J. Yao, J. C. Qin, H. Chen, F. Y. Wei, X. T. Liu, J. L. Wang and S. B. Wang, J. Hazard. Mater., 2015, 291, 28–37 CrossRef CAS PubMed.
- D. P. Li, S. X. Ge, J. Huang, J. J. Gong, T. X. Wang, P. Yan, G. B. Li and L. Y. Ding, Sep. Purif. Technol., 2014, 125, 216–222 CrossRef CAS PubMed.
- H. Liu, W. R. Cao, Y. Su, Z. Chen and Y. Wang, J. Colloid Interface Sci., 2013, 398, 161–167 CrossRef CAS PubMed.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, J. Phys. Chem. C, 2011, 115, 7355–7363 CAS.
- M. A. Butler, J. Appl. Phys., 1977, 48, 1914–1920 CrossRef CAS PubMed.
- W. J. Li, D. Z. Li, S. G. Meng, W. Chen, X. Z. Fu and Y. Shao, Environ. Sci. Technol., 2011, 45, 2987–2993 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O characteristic stretching of the free carboxylic group. While in the case of ZnTAPc–10% BFG composite, two new bands emerge at 1636 and 1260 cm−1 corresponding to the stretching bands of C
O characteristic stretching of the free carboxylic group. While in the case of ZnTAPc–10% BFG composite, two new bands emerge at 1636 and 1260 cm−1 corresponding to the stretching bands of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–N from the amide group,17 respectively. These results clearly indicate that the ZnTAPc molecules are covalently bound to the BFG by the amide linkage.
O and C–N from the amide group,17 respectively. These results clearly indicate that the ZnTAPc molecules are covalently bound to the BFG by the amide linkage.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C),23 respectively. For ZnTAPc–10% BFG, one component of N–C band shifted to lower binding energy by 0.9 eV while one component of N
C),23 respectively. For ZnTAPc–10% BFG, one component of N–C band shifted to lower binding energy by 0.9 eV while one component of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band shifted to higher binding energy by 0.8 eV, which indicate the charges transfer from ZnTAPc to BFG.
C band shifted to higher binding energy by 0.8 eV, which indicate the charges transfer from ZnTAPc to BFG.













