Functional properties of ZnCo2O4 nano-particles obtained by thermal decomposition of a solution of binary metal nitrates
C. R. Mariappan*a,
R. Kumara and
G. Vijaya Prakashb
aDepartment of Physics, National Institute of Technology, Kurukshetra, Haryana 136 119, India. E-mail: crmari2005@yahoo.com; Fax: +91 1744 238 050; Tel: +91 1744 233 498
bNanophotonics Laboratory, Department of Physics, Indian Institute of Technology-Delhi, New Delhi 110016, India
First published on 27th February 2015
Abstract
Spinel-type ZnCo2O4 nano-crystalline particles are prepared by direct thermal decomposition of a solution of binary metal nitrates at a relatively low temperatures (100 °C). Structural studies by X-ray diffraction and X-ray photoelectron spectroscopy reveal the predominant spinel crystal phase for ZnCo2O4. The high resolution transmission electron microscopy images with selected area diffraction patterns show that the spinal-type ZnCo2O4 nano-particles have a mean particle size of around 20 nm. Magnetic measurement reveals weak ferromagnetic properties for the spinel-type ZnCo2O4 nano-particles. The impedance spectroscopy measurement suggests a p-type nature for the prepared ZnCo2O4 nano-particles. The activation energies in Ar, air and O2 atmospheric conditions are found to be 0.46 eV, 0.43 eV and 0.35 eV respectively for temperatures below 200 °C. For temperatures greater than 200 °C, these samples show an intrinsic nature with activation energy of 0.57 eV for all atmospheric conditions. The ZnCo2O4 nano-crystals show a promising chemisorption process-related gas sensing behaviour for liquefied petroleum gas and they are investigated by DC and AC impedance measurements. A mechanism for the gas sensing behaviour of ZnCo2O4 is discussed.
1. Introduction
Nano-structured semiconducting ZnCo2O4 spinel-type oxides have been investigated intensively for various potential applications.1–8 In addition to the different potential applications, attention towards the cobalt based spinel-type ZnCo2O4 has increased very recently due to its p-type semiconducting behavior as a hole transport layer in organic photovoltaics (PVs).5 p-Type semiconducting oxides with high electrical conductivity, large work function and low synthesis/processing temperatures could have viable application as p-type electrodes in PVs. During these investigations, a wide variety of fabrication techniques have been developed, such as co-precipitation, sol–gel, combustion, micro-emulsion, hydrothermal, molten salt and Pechini polymerization methods to mention a few.3,7–11 The major benefit of a solution-based synthetic protocol is the molecular level mixing of metal ions which assists the construction of polycrystalline homogeneous nano-structures with enhanced physiochemical properties.Recently ZnCo2O4 nano-structured materials have been investigated as chemical sensors for hazardous gases such as SO2, CO, Cl2, NO2, CH3COOH, C2H5OH and LPG.7,10 It is fairly well known that the gas sensing mechanism of semiconductor metal oxides is mainly surface-controlled and the sensing behaviour is controlled by the transport properties of grain sizes and grain boundaries.12 Therefore the study of transport properties, their surface morphology dependence and gas/solid interactions have to be investigated to understand the gas sensing mechanism for nano-granular based devices. However, the majority of the gas sensing studies of ZnCo2O4 are based on bulk measurements such as DC resistance and hardly any reports are available on the effect of dynamically variable electrical quantities such as impedance and capacitance. The impedance spectroscopic method is a unique tool to study the nature of conduction processes and the mechanism of gas/solid interactions, which exploits the molecular dynamic relaxation processes involved during electrical injection.13–15
Most of the ultrafine powders or nano-structured ZnCo2O4 materials are obtained by heating the precursors between 280 °C to 600 °C.1–9 The focus of the present article is to propose a simple, inexpensive and effective synthesis of spinel-type ZnCo2O4 nano-particles by direct thermal decomposition of binary metal nitrate solutions at relatively low temperatures (100 °C) and to investigate the functional properties of the ZnCo2O4 nano-particles. Structural and optical properties of the samples are studied utilising thermogravimetric and differential thermal analyses (TG-DTA), powder X-ray diffraction (XRD), Fourier transformation infrared spectroscopy (FTIR), UV-visible (UV) absorption, photoluminescence (PL), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and high resolution transmission electron microscopy (HR-TEM) with selected area electron diffraction (SAED) pattern techniques. Magnetic behaviour is studied by vibrating sample magnetometer (VSM) measurement. Electrical properties and gas sensing behaviours are investigated by DC resistance and AC impedance measurement techniques at different temperatures.
2. Experimental
To obtain the ZnCo2O4 nano-particles, Zn(NO3)2·6H2O (1.203 g) powder is dissolved in ethanol (10 mL) and stirred for approximately 10 min to obtain a colorless solution. To the resulting solution, an ethanolic solution of Co(NO3)2·6H2O (2.355 g in 15 mL) is added drop wise and then rapidly stirred for more than six hours at room temperature (RT) to yield a deep purple colored solution. Then the solution is slowly heated to 40 °C, 60 °C, and 80 °C and maintained for 2 h at each temperature step with stirring. Then, the homogenous solution is allowed to dry at 100 °C for 2 h to obtain a black shiny powder. The obtained powders are calcined at various temperatures between 200 °C to 850 °C for 2 h for further structural characterization.Structural features are investigated by powder X-ray diffraction (XRD; Cu Kα radiation; λ = 1.541 Å) using a Siemens model-D500 X-ray diffractometer. Thermogravimetric and differential thermal analyses (TG-DTA) are performed for the sample using TA instruments model SDT 2960. The temperature was varied from room temperature to 1000 °C at a sweep heating rate of 5 °C min−1 in air. Fourier transform infrared spectra are recorded in the range of 4000–400 cm−1 using the KBr pellet technique using a Shimadzu FTIR-8700 spectrophotometer. For the UV-visible absorbance measurements, ethanolic solutions of powders are prepared by ultrasonication. The UV-visible absorption and photoluminescence (PL) spectra are recorded using Shimadzu UV-2600 absorption spectrophotometer and Shimadzu-2250 photoluminescence spectrometers respectively. The particle size and crystallite size of calcined powders are obtained from high resolution transmission electron microscopic (HR-TEM) images and selected area electron diffraction (SAED) pattern with a JEOL, (JEM 2100) electron microscope. Surface analysis of the pellet sample is carried out using X-ray photoelectron spectroscopy (XPS, SPECS) using monochromated Al Kα (1486.6 eV) radiation as an excitation source. The core level binding energy values are charge-corrected to the C 1s signal (284.6 eV).
The nano-crystalline ZnCo2O4 powders are coaxially pressed into a pellet and subjected to heating process at 750 °C for 10 h. In order to confirm the nano-structure of the sintered pellet, scanning electron microscopy (SEM) investigations are conducted on the cross section of a broken pellet using a JEOL Field Emission SEM model JSM-7500F. For impedance measurements, gold contacts on both faces of the sintered pellet are sputtered using a vacuum coater model BC 300. The impedance measurements are carried out on a Solartron, Model SI 1260, ac impedance analyzer over the frequency range 1 MHz–10 Hz at different temperatures between 50 °C to 400 °C under different controlled conditions such as air, argon and oxygen. For gas sensing studies, the pellet is housed in a home-made glass container of 750 mL capacity and provided with sample injection port, air inlet and air outlet at different temperatures.
3. Results and discussion
Fig. 1a shows the XRD patterns of nano-crystalline ZnCo2O4 powder samples calcined at different temperatures. All the recorded XRD patterns are in good agreement with the reported diffraction pattern of crystalline ZnCo2O4 (JCPDS #23-1390), suggesting the phase contains pure spinel structure and no secondary phases even at 750 °C calcination. The cell parameter (a = 8.0989 Å) estimated from the XRD pattern at 100 °C is in good agreement with the literature value of ZnCo2O4 nano-crystals obtained by combustion synthesis.9 Also it is comparable with the cell parameter (a = 8.093 Å) of Co3O4 powders obtained by the molten salt method.16 The cell parameter of ZnCo2O4 nano-crystals is found to be independent of calcination temperature. Increasing the calcination temperature to 850 °C leads to traces of secondary phases (Fig. 1a). At 850 °C, the substitution of Zn2+ by Co2+ is more favourable due to their similarity of ionic charge and radii, leading to formation of ZnO and Co3O4 secondary phases. The substitution of Zn2+ by Co2+ ions does not lead to a deformation in the measurable XRD pattern of the spinel Co3O4 due to its comparable cell parameter with ZnCo2O4. The average crystallite size was calculated by using Williamson–Hall analysis, which takes both the mean crystallite size D and strain ε effects into account:17
 | (1) |
 | ||
| Fig. 1 (a) XRD patterns of sample after calcination at different temperatures for 2 h. (b) TG and DTA curves for as synthesized sample at 100 °C. (c) FTIR spectrum of ZnCo2O4 nano-particles. | ||
The TG and DTA measurements depicted in Fig. 1b show the thermal evolution in air for the as prepared sample. The TG curve shows three weight losses, the initial loss (∼3%), up to 150 °C, is due to the evaporation of moisture or hydrated water. The second loss between 230 °C and 350 °C is most likely due to the evaporation of excess native nitrate moieties. Finally, the third weight loss (∼5%) accompanied by an endothermic peak around 875 °C is attributed to the reduction of the Co3+ state to the Co2+ state. The absence of any exothermic peak reveals that the spinel-type ZnCo2O4 nano-crystalline phase was formed during the synthesis (prepared at 100 °C) only, which is also evident from XRD studies (Fig. 1a).
Fig. 1c shows the FT-IR spectrum of ZnCo2O4 nanoparticles. The spectrum shows two strong absorption bands at 666 cm−1 and 570 cm−1. The peak at 666 cm−1 is ascribed to the stretching vibration mode of tetrahedrally coordinated metal (M) oxides (M–O) and the band at 570 cm−1 can be attributed to the octahedrally coordinated metal ions. This observation further supports the spinel crystal structure nature of the fabricated ZnCo2O4.2
The spinel-type ZnCo2O4 nanoparticles show good absorbance of light from 200 nm to 600 nm, as shown in Fig. 2a. The optical band gap energy, Eg, can be estimated by Tauc plots of expression, (αhν)n = K(hν − Eg), where hν is the photo energy, α is the absorption coefficient, K is a constant relative to the material, and n is 2 for a direct transition or 1/2 for an indirect transition. The (αhν)2 vs. hν curve for ZnCo2O4 nano-particles is shown inset in Fig. 2a. The estimated optical band gap energy of ZnCo2O4 nano-particles is 3.37 eV. Furthermore, there is one more absorption characteristic observed at a lower energy of 1.99 eV. This absorption band is ascribed to Co interatomic d–d transitions connected with a trigonal ligand field splitting,18 and suggests that some amount of the Co ions are interchanged with the tetrahedrally coordinated Zn ions. The Co ion can exist in this coordination since the ionic radius is similar to Zn ions.19,20 Photoluminescence spectra of ZnCo2O4 nano-particles with different excitation wavelengths are depicted in Fig. 2b within the spectral region of 200 nm–900 nm. For all excitation wavelengths, within the band edge, a single peak is observed at 3.41 eV (365 nm) and it is ascribed as near-band edge emission of the sample.
In order to establish the nano structure and the crystal phase nature of the synthesized ZnCo2O4, the HR-TEM and SEAD data were recorded and are reported in Fig. 3. The TEM images of ZnCo2O4 powder synthesised at 100 °C reveal an average particle size of ∼20 nm (Fig. 3). The SEAD pattern (inset Fig. 3a) of the sample prepared at 100 °C is clearly displaying diffraction rings with bright spots indicating the nano-crystalline nature of the sample. The diffraction rings are assigned to (111), (220), (311), (400), (511) and (440) planes, which are in good agreement with the XRD results (Fig. 1a). The close view HR-TEM images (Fig. 3c and d) depict well developed lattice fringes, which correlate well with the XRD results. The estimated d value of 2.46 Å corresponds to the (311) Miller indices of spinel-type ZnCo2O4. Fig. 3b and d show the TEM and SEAD pattern of a sample calcined at 750 °C for 2 h. The comparative results show that the crystallite size increases with the increase of calcination temperature and generates the well-defined shape of the particles with average diameter of ∼55 nm. The sizes of the crystallites are also in good agreement with the William–Hall XRD analysis estimations. The SEAD patterns (inset of Fig. 3a and b) and close view HR-TEM images (inset of Fig. 3c and d) of the sample prepared at 100 °C and calcined at 750 °C show similar crystal features. Therefore, it is possible to conclude that a temperature as low as 100 °C is the optimum condition for the formation of the nano-crystalline ZnCo2O4 phase.
The surface chemical compositions of ZnCo2O4 nanoparticle are identified by XPS analysis. Fig. 4a displays a survey spectrum of ZnCo2O4 nanoparticles calcined at 750 °C for 2 h and it reveals the characteristic peaks of Zn, Co, O, and C elements. Fig. 4b shows two strong peaks at 794.40 eV and 779.34 eV for Co 2p1/2 and Co 2p3/2 respectively, and confirms the presence of Co in a 3+ oxidation state.21 The high resolution Zn 2p spectrum (Fig. 4c) shows two peaks with binding energy values of 1044.05 eV and 1021.0 eV, assigned to Zn 2p1/2 and Zn 2p3/2 which show the presence of Zn in a 2+ oxidation state.10 The O 1s peaks at 529.33 eV and 531.18 eV correspond to the oxygen species in the spinel structure lattice and the oxygen in hydroxyl species adsorbed on the surface due to the ex situ measurement conditions (Fig. 4d) respectively.21 Thus XPS results, along with the other aforementioned studies, confirm the formation of ZnCo2O4 nano-particles with normal spinel structure.
 | ||
| Fig. 4 XPS spectra for the ZnCo2O4 nano-particles: (a) survey spectra and high-resolution (b) Zn 2p, (c) Co 2p, (d) O 1s spectra. | ||
Fig. 5 depicts the magnetization versus magnetic field (M − H) curve for the ZnCo2O4 nano-particles calcined at 750 °C for 2 h. The fine shape of the M − H curve is characteristic of a weak ferromagnetic behaviour, although paramagnetic behaviour has been reported for the bulk ZnCo2O4 ceramic.22 The coercive field (Hc) and remanent magnetization (Mr) from the M − H curve is found to be 0.10 kOe and 0.012 emu g−1 respectively as shown in the inset of Fig. 5. The smaller values of Hc and Mr prove that the ZnCo2O4 nano-particles possess weak ferromagnetic behaviour. The magnetization does not saturate for the maximum applied magnetic field 12 kOe and it represents the weak ferromagnetic ordering of the spins in the nano-particles which consists of small magnetic domains with random orientations.
An SEM photograph of the fractured surface of the ZnCo2O4 pellet sintered at 750 °C for 10 h is shown in Fig. 6. The shape of the grains in the sintered pellet is close to circular and is similar to the TEM result (Fig. 3b). This SEM image also reveals that the grains are well connected which would facilitate the conduction pathway for the charge carriers. Also the EDX result shows the atomic ratio of Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co is 1
Co is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
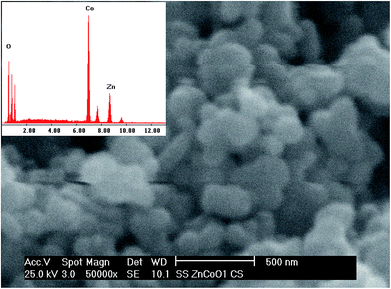 | ||
| Fig. 6 SEM micrograph with EDX information of the fracture surface of the ZnCo2O4 nano-crystalline pellet sintered at 750 °C for 2 h. | ||
Complex impedance spectra of nano-crystalline ZnCo2O4 pellet recorded at different temperatures in air are shown in Fig. 7a. A single semi-circle profile is observed for different temperatures and the diameter of the semicircle is observed to decrease with temperature. Fig. 7b shows the Nyquist plots of AC impedance of a sample at 150 °C with different ambient conditions such as air, Ar and O2. Based on the measured impedance data, the representative electrical equivalent circuit was established as shown in the inset Fig. 7a. In the equivalent circuit elements, the resistance of the sample is represented as R, the capacitance is denoted as “C” and inductance is represented as L due to coaxial cable connected to the impedance analyzer. The measured impedance spectra were fit to the equivalent circuit using Zview® software.
The R of the sample decreases with increase in temperature due to increase of the mobility of the charge carrier and the number density (Nd), whereas the C of the sample slightly increases with temperature. The fitted capacitance values of the nano-crystalline ZnCo2O4 pellet in the presence of air are 39.7 pF at 50 °C, 42.5 pF at 100 °C, 43.8 pF at 150 °C, 45.1 pF at 200 °C, 45.9 pF at 250 °C and 46.8 pF at 300 °C. The chemisorbed oxygen molecules are converted into oxide ions by capturing the free electrons and create the holes on the valence band. This creates the negatively charged layer over the grain and also the positively charged grain interior.14 This space charge layer is a kind of capacitor, since the electrical charge exists locally at the grain boundary.23 The relaxation time (τ = RC) of ZnCo2O4 decreases with temperature due to the decrease of R.
A deeper insight into the electrical properties of the nano-crystalline ZnCo2O4 pellet was obtained from the spectroscopic plots of electric modulus (M′′) and impedance (Z′′) versus frequency (ν) at 150 °C (Fig. 7c) in air, Ar and O2 atmosphere conditions. The peak frequency of both the curves (Z′′ and M′′) is the same, which indicates that the impedance peak associated with the RC element is responsible for the modulus peak. The associated capacitances are of the order of pF and it is characteristic of the bulk sample. The maximum of M′′ is almost same at 150 °C in air, Ar and O2, whereas the maximum of Z′′ decreases with resistance at 150 °C in air, Ar and O2. The relaxation frequency (ν = 1/2πRC) of ZnCo2O4 is high in the presence of O2 compared to in the presence of Ar and air. This reveals that the relaxation process of the charge carrier is distorted by the different atmospheric conditions for ZnCo2O4 nano-crystals.
The DC conductivity σdc was calculated by multiplying the inverse of resistance (R−1) with the ratio of thickness to area of the sample pellet. The estimated DC conductivity at 150 °C (σdc = 2.85 × 10−6 S cm−1) of ZnCo2O4 in the presence of O2 atmosphere is higher than the values observed in the presence of air (1.45 × 10−6 S cm−1) and Ar atmosphere (7.88 × 10−7 S cm−1). It can be speculated that when the partial pressure of O2 is increased, a larger number of chemisorbed oxygen molecules can be converted into either O2− or O− by taking the free electron from the ZnCo2O4 and creating more holes on the valance band. As a result of the increasing number of hole charge carriers, the conductivity of the sample is enhanced. The overall results suggest that the fabricated ZnCo2O4 is of p-type, having holes as the major charge carriers for electrical conduction.
Results of the temperature dependence of the DC conductivity σdc(T) in different ambient conditions are shown in Fig. 7d. The σdc(T) plots of the nano-crystalline ZnCo2O4 pellet show behavior similar to that of conventional extrinsic semiconductors. The σdc(T) plots are separated into two different sections: (I) 50 °C–200 °C and (II) 200 °C–400 °C. The σdc(T) of ZnCo2O4 in section I is of extrinsic origin from the acceptor level, while the σdc(T) in section II is of intrinsic origin. The activation energies (EA) for DC conductivity of ZnCo2O4 nano-particles are obtained from the Arrhenius equation. The estimated activation energies (EA) of the sample are found to be 0.46 eV, 0.43 eV and 0.35 eV for the temperature range of 50 °C–200 °C (extrinsic origin) in Ar, air and O2 atmospheres respectively. A moderate difference in activation energies under various atmospheric conditions is due to the existence of a different local electrical field or different size of energy barrier locally at the grain boundary. For the temperature range above 200 °C (intrinsic origin), the activation energy is observed to be 0.57 eV in all the atmospheric conditions (O2, Ar and air). This reveals that the energy barrier for electrical conduction is independent of atmospheric conditions above 200 °C. The thermal energy of ≥42 meV is indeed sufficient to reduce/remove the different size of the energy barrier that exists locally at the grain boundary due to the different ambient conditions. Hymavathi et al. have reported that the EA of ZnCo2O4 bulk ceramic is 1.01 eV for the temperature range of 50 °C to 500 °C in air.24 Kim et al. have observed that the EA of ZnCo2O4 thin film is 42 meV for the temperature range of −93 °C to 200 °C.25 The activation energy observed for the present nano-crystalline ZnCo2O4 pellet is lower than the ZnCo2O4 bulk ceramic24 and higher than the ZnCo2O4 thin film.25
The gas sensing behaviour for spinel-type ZnCo2O4 nano-crystals was studied by the DC resistance measurement technique as a function of temperature for 500 ppm of LPG. The most favourable response was found at a temperature of 365 °C as shown in Fig. 8a. The resistance of the sample increases with increasing concentration of LPG. The response and recovery times are defined as the time taken to reach 90% of the final resistance value. The response and recovery time for LPG is found to be 85 s and 95 s respectively at 365 °C. Sensitivity for a given concentration of LPG, was calculated by the ratio of Rg/Ra where Ra is the resistance of the sensor in air and Rg is the resistance of the sensor in the presence of the LPG. Gas sensitivity increases with concentration at 365 °C as shown in the inset of Fig. 8a.
Fig. 8b describes the Nyquist plots of AC impedance of ZnCo2O4 at 365 °C in the presence of air and in the presence of 100 ppm of LPG. A single half semi-circle is obtained which increased in the presence of analyte gas. In order to understand the changes of resistance and capacitance of the sample with LPG, we measured them as a function of time at a fixed ac frequency, which was close to the relaxation frequency of the sample (ν = 1/2πRaCa) as shown in Fig. 8c. The gas sensing mechanism has a surface controlled nature, in which the grain size, surface states and the density of chemisorbed oxygen ions play significant roles.10 As discussed previously, when the ZnCo2O4 nano-crystals are exposed to air at elevated temperature, chemisorbed oxygen molecules can capture the free electrons and create holes on the valence band. This can be described by:
| O2 (gas) ↔ O2 (ads) | (2) |
| O2 (ads) + 4e−↔ 2O2− (ads) | (3) |
The stabilization of the surface resistance and capacitance is dependent on the equilibrium of the chemisorptions process (eqn (3)). Such stabilised chemisorbed surface resistance can be changed by any process that disturbs the equilibrium. The free electrons are released when the reducing gases (LPG contains CH4, C3H8, C4H10 etc.) interact with the chemisorbed oxide ions of the ZnCo2O4 surface. This eventually decreases the hole concentration and consequently results in the increase of resistance. However, the change in the capacitance values is not clearly accounted for in the case of the LPG sensor (Fig. 8c). Since the capacitance is directly proportional to the dipole moment, the relatively smaller values of LPG (μ = 0.084 for propane, μ = 0 for butane, μ = 0.13 for isobutene) mean that hardly any changes are observed. To check the cross sensitivity, the AC impedance was measured towards 5000 ppm of H2 at 365 °C as shown in Fig. 8d. Considerable changes to AC impedance or resistance and capacitance for ZnCo2O4 were not observed with 5000 ppm of H2.
4. Conclusions
Nano-structured ZnCo2O4 powders were successfully synthesized at relatively low temperatures (100 °C). The present XRD and HR-TEM studies revealed that the ZnCo2O4 nano-particles exist in the pure spinel-type phase structure with a cell parameter of a = 8.0989 Å. The average crystallite size is found to be approximately 20 nm for the sample prepared at 100 °C. The optical band-gap energy of the ZnCo2O4 nano-particles is found to be 3.37 eV. Magnetic measurements revealed weak ferromagnetic properties. Activation energies of DC conductivity are found to be 0.46 eV, 0.43 eV and 0.35 eV in Ar, air and O2 respectively for the temperature range of 50 °C–200 °C and 0.57 eV in all atmospheric conditions (Ar, air and O2) for the temperature region of 220 °C–400 °C. The gas sensing characteristics of nano-sized ZnCo2O4 are examined by DC and AC impedance measurements. The most favourable response was found at a temperature of 365 °C towards LPG and the sensitivity is observed to increase with concentration. The changes on the DC resistance/real part of impedance of the sample in the presence of LPG are related to chemisorption processes and governed by the number density of charge carriers.Acknowledgements
The financial support by SERB-DST (India) research grant no. SERB/F/5418/2014-15 is gratefully acknowledged. We are grateful to A. Sreeramamurthy and P.C. Clinsha for the XPS measurements. We thank profusely Scott E. Harpstrite and Dr S. Jothilingam, University of St. Louis, USA and Dr C. R. Ramanathan for critical reading of the manuscript. The work is also partly supported by High-Impact research initiative of IIT Delhi.References
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, Chem. Rev., 2013, 113, 5364 CrossRef CAS PubMed.
- W. Luo, X. Hu, Y. Sun and Y. Huang, J. Mater. Chem., 2012, 22, 8916 RSC.
- M. V. Reddy, K. Y. H. Kenrick, T. Y. Wei, G. Y. Chong, G. H. Leong and B. V. R. Chowdari, J. Electrochem. Soc., 2011, 158, A142 Search PubMed.
- M. Davis, C. Gumeci, B. Black, C. Korzeniewski and L. Hope- Weeks, RSC Adv., 2012, 2, 2061 RSC.
- N. H. Perry and T. O. Mason, J. Am. Ceram. Soc., 2013, 96, 966 CrossRef CAS PubMed.
- Y. Qiu, S. Yang, H. Deng, L. Jin and W. Li, J. Mater. Chem., 2010, 20, 4439 RSC.
- X. Niu, W. Du and W. Du, Sens. Actuators, B, 2004, 99, 405 CrossRef CAS PubMed.
- Z. Jia, D. Ren, A. Wang and R. Zhu, Appl. Surf. Sci., 2013, 270, 312 CrossRef CAS PubMed.
- Y. Sharma, N. Sharma, G. V. S. Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2855 CrossRef CAS.
- S. Vijayanand, P. A. Joy, H. S. Potdar, D. Patil and P. Patil, Sens. Actuators, B, 2011, 152, 121 CrossRef CAS PubMed.
- B. Liu, J. Zhang, X. Wang, G. Chen, D. Chen, C. Zhou and G. Shen, Nano Lett., 2012, 12, 3005 CrossRef CAS PubMed.
- G. Korotcenkov, S.-D. Han, B. K. Cho and V. Brinzari, Crit. Rev. Solid State Mater. Sci., 2009, 34, 1 CrossRef CAS.
- Z. Ling, C. Leach and R. Freer, Sens. Actuators, B, 2002, 87, 215 CrossRef CAS.
- C. Malagu, M. C. Carotta, S. Gherardi, V. Guidi, B. Vendemiati and G. Martinelli, Sens. Actuators, B, 2005, 108, 70 CrossRef CAS PubMed.
- A. Labidi, C. Jacolin, M. Bendahan, A. Abdelghani, J. Guerin, K. Aguir and M. Maaref, Sens. Actuators, B, 2005, 106, 713 CrossRef CAS PubMed.
- M. V. Reddy, Z. Beichen, L. J. Nicholette, Z. Kaimeng and B. V. R. Chowdari, Electrochem. Solid-State Lett., 2011, 14, A70 CrossRef PubMed.
- G. K. Williamson and W. H. Hall, Acta Metall., 1953, 1, 22 CrossRef CAS.
- K. Samanta, P. Bhattacharya and R. S. Katiyar, Appl. Phys. Lett., 2005, 87, 101903 CrossRef PubMed.
- R. D. Shannon and C. T. Prewitt, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 925 CrossRef CAS.
- M. Dekkers, G. Rijnders and D. H. A. Blank, Appl. Phys. Lett., 2007, 90, 021903 CrossRef PubMed.
- B. Varghese, T. C. Hoong, Z. Yanwu, M. V. Reddy, B. V. R. Chowdari, A. T. S. Wee, T. B. C. Vincent, C. T. Lim and C. H. Sow, Adv. Funct. Mater., 2007, 17, 1932 CrossRef CAS.
- P. Cossee, Recl. Trav. Chim. Pays-Bas, 1956, 75, 1089 CrossRef CAS.
- M. Labeau, U. Schmatz, G. Delabouglise, J. Roman, M. Vallet-Regi and A. Gaskov, Sens. Actuators, B, 1995, 26, 49 CrossRef CAS.
- B. Hymavathi, B. R. Kumar and T. S. Rao, AIP Conf. Proc., 2012, 1461, 299 CrossRef CAS PubMed.
- H. J. Kim, I. C. Song, J. H. Sim, H. Kim, D. Kim, Y. E. Ihm and W. K. Choo, Appl. Phys. Lett., 2004, 95, 7387 CAS.
| This journal is © The Royal Society of Chemistry 2015 |





