Beckmann rearrangement reaction of cyclohexanone oxime in sub/supercritical water: byproduct and selectivity
Yang Li,
Kai Wang*,
Kang Qin and
Tao Wang*
State Key Laboratory of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China. E-mail: taowang@tsinghua.edu.cn; kaiwang@tsinghua.edu.cn; Tel: +86 10 62784877 Tel: +86 10 62788568
First published on 3rd March 2015
Abstract
As an important green synthesis technology of caprolactam, the Beckmann rearrangement reaction of cyclohexanone oxime using high temperature and pressure water as the reaction media was carefully studied with a microreactor. An important byproduct – acetamide was confirmed with a selectivity higher than 40% in the subcritical water, which was more serious than the reported cyclohexanone. The effects of phase state and operating conditions, such as reaction temperature, pressure, flow rate ratio and reactant residence time, on the cyclohexanone oxime conversion and caprolactam selectivity were carefully analyzed, and the results showed that keeping the reaction close to the supercritical state was critical for the preparation of caprolactam.
1 Introduction
The Beckmann rearrangement reaction of cyclohexanone oxime is an important chemical process for the preparation of caprolactam,1 the monomer of Nylon-6, as shown in Scheme 1. In the industrial process, more than 90% caprolactam is synthesized from the conventional cyclohexanone route catalyzed by fuming sulfuric acid.2 As a strong corrosion process, it still has some drawbacks, such as the large amount of byproduct ammonium sulfate, it is unfriendly for environment and has serious safety problems.3,4 Since caprolactam is an important chemical product with growing demand, there have been lots of studies on developing new Beckmann rearrangement processes. In the last 10 years, a large number of solid-acid catalysts, such as B2O3,5 Nb2O5,6 and zeolite7 have been investigated to proceed the Beckmann rearrangement reaction at the vapor state, which are usually operated at a temperature higher than 300 °C and nearly 0.1 MPa pressure. Besides, the liquid Beckmann rearrangement reaction using organic acid catalyst is another hot-point area.8 For example, Ronchin et al.9,10 reported 92% selectivity of caprolactam was achieved, catalyzed by trifluoroacetic acid in aprotic solvents such as toluene.The Beckmann rearrangement reaction in high temperature and pressure water is another hot point for the synthesis of caprolactam, which attracts rising interest in recent years. Since the thermodynamic properties of water can be easily changed with the variation of pressure and temperature,11–13 nonpolar organic compounds become miscible in supercritical water. The sub/supercritical water also works as a catalyst in some reactions, such as methyl allyl aryl ether Claisen rearrangement reaction at 5 MPa and 265 °C14 and Friedel–Crafts alkylation reaction of phenol at 275 °C in the absence of any acid catalyst.15 Using supercritical water instead of organic solvent, organic catalyst and fuming sulfuric acid catalyst offers a great opportunity to prevent environment problems and the generation of useless ammonium sulfate. In the area of cyclohexanone oxime Beckmann rearrangement in supercritical water, Ikushima et al.16–19 have reported some prospective works. In a preliminary batch reaction study, they found when the cyclohexanone oxime aqueous solution was heated up to the critical point (374.2 °C, 22.13 MPa), caprolactam can be measured, but the yield was only 1.9%. Lots of cyclohexanone was obtained as the main byproduct generated from the hydrolysis of cyclohexanone oxime. The authors believed the temperature raising took so much time resulted the yield of cyclohexanone during the subcritical water state, where the ion product (Kw) was much bigger than the ambient water and the cyclohexanone oxime became unstable. A supercritical water microreactor system with instant heating function according to fast mixing of hot water and cool cyclohexanone oxime solution was therefore invented, achieving nearly 80% yield of caprolactam at 40 MPa and 375 °C. Then, the system achieved nearly 100% yield of caprolactam by using some dilute acid as the catalyst. Cyclohexanone was the unique reported byproduct in their papers and the use of dilute acid did not realize a genuine green synthesis. Further work on the byproduct generation rules in sub/supercritical water is still an important issue in this area.
In this paper, we developed a similar work using a capillary microreaction system with fast heating and fast quenching functions. Considering the subcritical phase was unavoidable in the caprolactam synthesis, which was important for the reaction yield, we paid more attention in this part and another byproduct acetamide was discovered, which was ignored in previous reports. The variation rules of cyclohexanone oxime conversion and caprolactam selectivity were carefully analysis and the importance of mixing and phase state were also discussed.
2 Experimental
2.1 Experimental setup and procedures
The Beckmann rearrangement reaction was conducted in a capillary microreaction setup, as shown in Fig. 1. It was organized by three feeding pumps, a heating oven, a heat preservation room, two micromixers, a reaction tube, three temperature sensors, a cooler, a filter, a back-pressure valve and a gas–liquid separator. Among those devices, the most important parts were the two T-type micromixers, which inner diameter was 0.75 mm. In the experiment, a stream of water at ambient temperature was first heated into the oven using a 2 m long, 1/8 inch diameter Hastelloy C-276 tube as the pre-heater. The max temperature of this hot water was about 500 °C. A heating plate made by copper was placed in the oven and the pre-heating tube was embedded in the copper plate to deliver heat. A stream of cyclohexanone oxime aqueous solution at ambient temperature was then mixed with the high temperature supercritical water out of the pre-heating tube in the first T-type micromixer and the cyclohexanone oxime solution was quickly heated to a reaction temperature, ranged from 320 to 420 °C. The mixture was then introduced into a 1/16 inch reaction tube, whose inner diameter was 0.6 mm. The temperature at the reaction tube inlet was monitored by a sensor and its value was used to adjust the temperature of heat preservation room with a deviation less than 10 °C. According to the temperature values after the first micromixer, the mixing of hot water and cold cyclohexanone oxime aqueous solution was almost complete, which fixed heat balance, and this phenomenon accorded to our previous studies20,21 on micromixing process, which mixing time was in several milliseconds. In order to give a strict control of the reaction time, the reacting mixture was quenched by mixing with another stream of cold water at the second T-type micromixer, which was not adapted in Ikushima's work.17,18 The temperature of this new mixture was also monitored by a temperature senor, whose values varied from 90 to 160 °C. The mixture was further cooled to room temperature in a cooler, who had a cooling tube with inner diameter 2 mm in it. The solution was finally filtered and released to the gas–liquid separator in order to collect the liquid samples. The reaction pressure was controlled by a back-pressure regulator from 16 to 32 MPa. In every experiment, pressure test using a flow of pure water was first made to exclude leaking, and then the heaters and cooler were turned on. As the system temperature reached the set value, the cyclohexanone oxime aqueous solution was pumped in, instead of the pre-fed water. 20 min was needed for the system reaching a steady state, and samples were collected in 3 min.2.2 Materials and analysis
In the experiment, the reactant cyclohexanone oxime (CHO) and the byproduct cyclohexanone (OH) were purchased from Alfa Aesar Co., Ltd. The caprolactam (CPL) was purchased from Aladdin Co., Ltd. Chromatographically pure solvents used in analysis (acetonitrile, chloroform) at were provided by Beijing Chemical Works. Ultrapure water (18.2 MΩ cm) was used as the reaction medium. The soluble O2 in water was removed by 30 min N2 bubbling. Since the concentration of cyclohexanone oxime used in the reaction was 1 g L−1, the physical properties of reacting solution was evaluated by pure water, which data are published on the web site of US National Institute of Science and Technology (NIST, http://webbook.nist.gov). The residence time in the reaction tube between two micromixers was calculated from the volume of the reactor and the mass velocity of the mixture as the following equation:
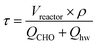 | (1) |
 | (2) |
 | (3) |
Samples of product from the outlet of microreaction system were mainly analyzed by a high performance liquid chromatograph (HPLC, Agilent 1260, wavelength of UV detector: 201 nm, mobile phase: 35% acetonitrile and 65% DI water, flow rate: 0.5 mL min−1). What's more, some samples were extracted by chloroform 3 times, and then the organic phase was analyzed by a gas chromatography (GC, Agilent 7890A, FID detector, gas: N2; injection temperature: 250 °C; column temperature: 170 °C; detector temperature: 240 °C) in order to analyze the selectivity of cyclohexanone. A gas chromatography-mass spectrometry (GC-MS, Thermo Fisher) was used to confirm the main byproduct using a 30 m × 0.25 m × 0.25 mm nonpolar capillary column and a flame ionization detector. The temperature gradient in operation was 10 °C min−1 from 60 to 300 °C and the retention time was 20 min. The ion source temperature was 250 °C and the electron beam energy was 70 eV.
3 Results and discussion
3.1 Stability of caprolactam
In order to discuss the Beckmann rearrangement reaction of cyclohexanone oxime, the stability of caprolactam in subcritical and supercritical water was an important issue, since it might be open loop hydrolyzed and the product 6-aminocaproic acid was hard to analysis by the UV detector in HPLC. An aqueous solution of caprolactam was carried out first, instead of the cyclohexanone oxime aqueous solution. Relative errors of caprolactam concentration in Table 1 showed that almost none hydrolyzation took place in a short residence time less than 2.5 s. So the caprolactam produced by the Beckmann rearrangement reaction was sufficiently stable in both subcritical and supercritical water.| T (°C) | p (MPa) | ρ (kg m−3) | ‖Wmix − W‖/Wmix | τ (s) |
|---|---|---|---|---|
| a A stream of hot supercritical water (4 g min−1) and a stream of caprolactam aqueous solution (1 g min−1, WCPL = 0.9 g L−1) at ambient temperature were mixed in the experiment. Then the mixed solution was mixed with another ambient water (9.99 g min−1) and the caprolactam concentration in the final mixture was Wmix. The caprolactam concentration in the outlet solution, W, is analyzed by a HPLC with instrumental error less than 5%. | ||||
| 164 | 30 | 920 | 1.29% | 2.5 |
| 238 | 30 | 841 | 2.25% | 2.3 |
| 343 | 30 | 662 | 0.12% | 1.8 |
| 366 | 30 | 594 | 1.10% | 1.6 |
| 372 | 30 | 571 | 1.97% | 1.5 |
| 390 | 30 | 371 | 2.23% | 1.0 |
3.2 Byproduct analysis
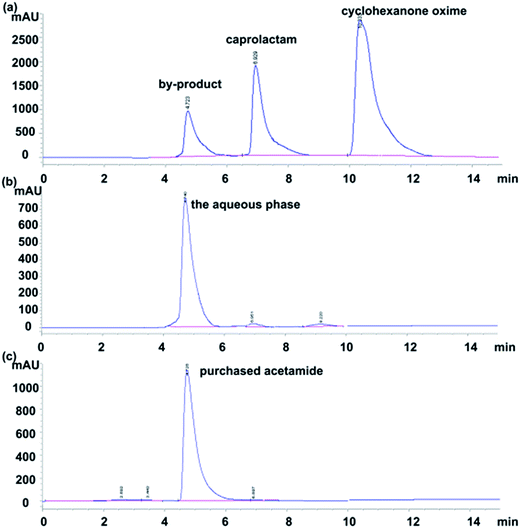
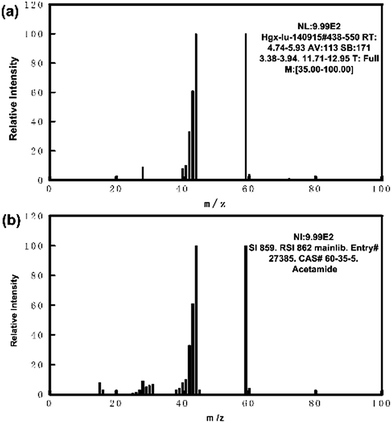
The sample of Beckmann rearrangement reaction of cyclohexanone oxime collected after the experiment was analyzed by HPLC firstly. There were three high peaks in our results as shown in Fig. 2(a). The second peak was caprolactam and the third one was cyclohexanone oxime, which were easily certified. The retention time of reported byproduct cyclohexanone was at 13 min, which is not obvious in our HPLC spectra, as shown in Fig. 2(a), but its retention time was confirmed by a purchased cyclohexanone sample in our HPLC. In order to figure out the undefined byproduct of the first peak, several 25 mL samples were extracted by chloroform (25 mL) 3 times at 40 °C. Then, the products were separated into two phases in a separating funnel and the aqueous phase was analyzed by HPLC again and a typical result is given by Fig. 2(b). This result shows only the undefined byproduct exits in the residual water phase. The organic chloroform phase was then analyzed by a GC in order to figure out the selectivity of cyclohexanone. The residual aqueous phase was then completely evaporated and collected in a rotary evaporator at 50 °C. The collected samples were dissolved in methanol, and the methanol solution was then analyzed by a GC-MS in order to figure out the residual composition.Fig. 3(a) shows the mass spectrogram of the byproduct in the methanol solution and Fig. 3(b) shows the standard sample of acetamide in methanol solution. The mass spectrogram of the unknown byproduct is in fair agreement with the purchased acetamide. HPLC spectrogram of the sample attained from Beckmann rearrangement reaction in Fig. 2(a) is also in accordance with the purchased acetamide solution as shown in Fig. 2(c). Thus, the main byproduct in our Beckmann rearrangement reaction is confirmed to acetamide. The results obtained by GC of the organic phase tell us there was a little cyclohexanone produced in our experiment with a selectivity less than 10% and the selectivity changed a little at different reaction conditions. Limited by the lack of the mechanism theory, an explanation was proposed according to the reported mechanism in literature.22 As shown in Scheme 2, during the reaction, the first step is the initial protonation of the oxygen atom in cyclohexanone oxime in the supercritical water, then the rearrangement occurs. But when this process is operated in subcritical water, the carbon ring becomes unstable for the high ionic strength, and one result is the broken of ring, producing a nitrilium ion and leading to the yield of the acetamide. When doing the GC-MS, we also found some other compounds in our sample, such as the methyl 2,2-dimethoxyacetate (CAS:89-91-8). However, the area ratio of peaks acetamide to methyl 2,2-dimethoxyacetate was nearly 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, thus there was very little methyl 2,2-dimethoxyacetate in our sample. What's more, the purity of cyclohexanone oxime purchased from Alfa Aesar Co., Ltd was 97%. One possible for the existing methyl 2,2-dimethoxyacetate was from the source material rather than reaction. So in this paper we will not discuss other byproduct any more.
1, thus there was very little methyl 2,2-dimethoxyacetate in our sample. What's more, the purity of cyclohexanone oxime purchased from Alfa Aesar Co., Ltd was 97%. One possible for the existing methyl 2,2-dimethoxyacetate was from the source material rather than reaction. So in this paper we will not discuss other byproduct any more.
3.3 Effects of operating conditions
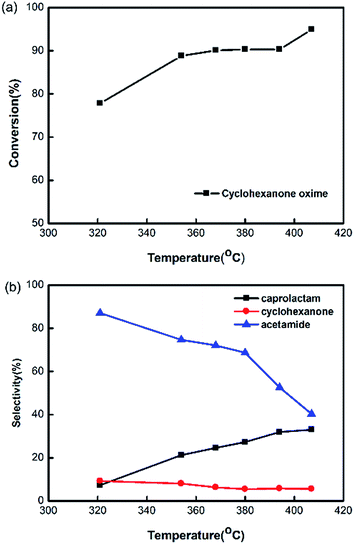
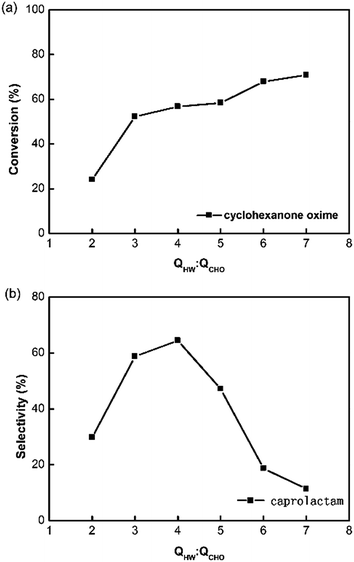
As the thermodynamics properties of water varies seriously with the temperature, it is necessary to discuss the effect of temperature on the reaction performance. The experiment was carried out in subcritical and supercritical water at 30 MPa by setting different temperatures of the pre-heater. Fig. 4 shows that the reaction temperature measured by the sensor in the reaction tube plays a great role. The conversion of cyclohexanone oxime rises from 78% to 95% with the increase of temperature. The selectivity of caprolactam rises from 8% to 38% with the increase of temperature, and the selectivity of acetamide decreases from 77% to 38%. While, the selectivity of cyclohexanone is less than 10% with little changes at different temperatures. These results mean that the supercritical condition is more favorable for the product of caprolactam. It is proved that Beckmann rearrangement reaction of cyclohexanone oxime followed a proton-catalyzed reaction mechanism.19,22 At higher temperature, the non-polar solvent environment would contribute to the breakdown of H–O bond, enhancing the generation of caprolactam.The reaction performance was also affected by the proportion of the hot water and cyclohexanone oxime solution, which changed the reaction temperature too. In the experiment, the flow rate of cyclohexanone oxime solution was constant at 1 mL min−1 and the cooling water in the second micromixer was the maximum value of our pump 9.99 mL min−1. We change the reaction temperature by varying the flow rate ratio of hot water and cyclohexanone oxime solution in this experiment, which was different from changing the temperature of the pre-heater in the last experiment. The results in Fig. 5 show that the flow rate ratio have an optimum for the yield of caprolactam. When the flow rate is small, the reaction temperature was low. Side reaction were easily proceeded in subcritical state as the result in Fig. 4. When the flow rate is high, a potential reason for the low selectivity is that the mixing between supercritical water and ambient solution is not good at extreme flow rate ratio.23 So the flow ratio of the ambient water and cyclohexanone oxime aqueous solution should have an optimum in the reaction.
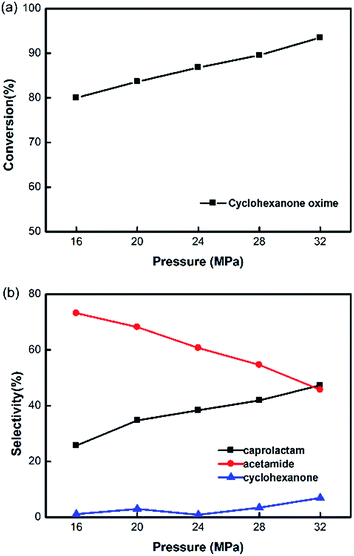
Considering the high dilatation coefficient of water with the increase of pressure, a series of experiments were carried out to study the influence of pressure at a given reaction temperature of 400 °C. The selectivity of caprolactam in Fig. 6 increases with the pressure rising, which reaches 48% at 32 MPa. It may be more enhanced at a higher pressure, but we could not verify the prediction because of the limitation of our pumps. In this reaction, pressure proved to sustain the formation of caprolactam. The ionic product (Kw) of H2O increases with the increasing of pressure at 400 °C. When coming to the supercritical condition, the increasing H+ concentration in solvent may explain the increase of the yield of caprolactam, since it has been demonstrated by Ikushima et al.17 that the addition of dilute acid aqueous solution could contribute to the yield.
Another research point on the Beckmann rearrangement reaction is to explore the influence of residence time on the cyclohexanone oxime conversion and caprolactam selectivity. A simple way of adjusting the residence time is changing the length of the reaction tube. So we carried out the experiments at different temperatures and 30 MPa. The results are shown in Fig. 7. The residence time exerts a great influence on the conversation of cyclohexanone oxime, which increased with the increasing residence time at a constant temperature. 93% of the conversation could be achieved at 400 °C in less than 3.5 s. The selectivity of caprolactam decreases with the rising of residence time and this result show the advantage of caprolactam generation at the early state of this reaction.
4 Conclusions
In this work, a new byproduct acetamide is found when investigate the Beckmann rearrangement reaction in subcritical and supercritical water. We also find that the phase state of water is important for the yield of caprolactam. A selectivity of 50% caprolactam and 90% conversation of cyclohexanone oxime could be achieved within less than 0.75 s at 32 MPa and 400 °C. It is demonstrated that high temperature and pressure improve the selectivity of caprolactam, but the optimized value still allows further studies.Acknowledgements
We would like to acknowledge the supports from the National Natural Science Foundation of China (91334201, U1463208) and the State Key Lab of Chemical Engineering of China (SKL-ChE-12T01) on this work.References
- G. Dahlhoff, J. Niederer and W. F. Hoelderich, Catal. Rev.: Sci. Eng., 2001, 43, 381–441 CAS.
- C. Yan, J. Fraga-Dubreuil, E. Garcia-Verdugo, P. A. Hamley, M. Poliakoff, I. Pearson and A. S. Coote, Green Chem., 2008, 10, 98 RSC.
- K. T. Zuidhof, M. de Croon and J. C. Schouten, AIChE J., 2010, 56, 1297–1304 CAS.
- J. S. Zhang, K. Wang, Y. C. Lu and G. S. Luo, AIChE J., 2012, 58, 3156–3160 CrossRef CAS.
- D. S. Mao, Q. L. Chen and G. Z. Lu, Appl. Catal., A, 2003, 244, 273–282 CrossRef CAS.
- M. Anilkumar and W. F. Hoelderich, J. Catal., 2012, 293, 76–84 CrossRef CAS PubMed.
- G. P. Heitmann, G. Dahlhoff and W. F. Holderich, J. Catal., 1999, 186, 12–19 CrossRef CAS.
- S. R. Narahari, B. R. Reguri and K. Mukkanti, Tetrahedron Lett., 2011, 52, 4888–4891 CrossRef CAS PubMed.
- L. Ronchin and A. Vavasori, J. Mol. Catal. A: Chem., 2009, 313, 22–30 CrossRef CAS PubMed.
- L. Ronchin, A. Vavasori and M. Bortoluzzi, Catal. Commun., 2008, 10, 251–256 CrossRef CAS PubMed.
- D. Broll, C. Kaul, A. Kramer, P. Krammer, T. Richter, M. Jung, H. Vogel and P. Zehner, Angew. Chem., Int. Ed., 1999, 38, 2999–3014 CrossRef CAS.
- E. Kiranand and J. F. Brennecke, ACS Symp. Ser., 1993, 514, 1–8 CrossRef PubMed.
- S. Marre, Y. Roig and C. Aymonier, J. Supercrit. Fluids, 2012, 66, 251–264 CrossRef CAS PubMed.
- H. Kawanami, M. Sato, M. Chatterjee, N. Otabe, T. Tuji, Y. Ikushima, T. Ishizaka, T. Yokoyama and T. M. Suzuki, Chem. Eng. J., 2011, 167, 572–577 CrossRef CAS PubMed.
- P. E. Savage, Chem. Rev., 1999, 99, 603–621 CrossRef CAS PubMed.
- Y. Ikushima, K. Hatakeda, O. Sato, T. Yokoyama and M. Arai, J. Am. Chem. Soc., 2000, 122, 1908–1918 CrossRef CAS.
- Y. Ikushima, K. Hatakeda, M. Sato, O. Sato and M. Arai, Chem. Commun., 2002, 2208–2209 RSC.
- Y. Ikushima, O. Sato, M. Sato, K. Hatakeda and M. Arai, Chem. Eng. Sci., 2003, 58, 935–941 CrossRef CAS.
- Y. Masuda, A. Suzuki and Y. Ikushima, Int. J. Heat Mass Transfer, 2006, 33, 419–425 CrossRef CAS PubMed.
- J. S. Zhang, K. Wang, Y. C. Lu and G. S. Luo, Chem. Eng. Process., 2010, 49, 740–747 CrossRef CAS PubMed.
- Z. D. Liu, Y. C. Lv, J. W. Wang and G. S. Luo, Chem. Eng. J., 2012, 181–182, 597–606 CAS.
- Y. Yamaguchi, N. Yasutake and M. Nagaoka, J. Mol. Struct., 2003, 639, 137–150 CrossRef CAS PubMed.
- X. Y. Lin, K. Wang, J. S. Zhang and G. S. Luo, Chem. Eng. Sci., 2015, 127, 60–71 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |