Extraction and GC-MS analysis of phthalate esters in food matrices: a review
Abstract
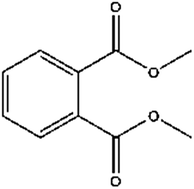
According to the Scopus database, using “phthalate” and “GC” as keywords, 758 papers have been found between 1990 and 2014, showing strong and increasing interest in this class of compounds from the scientific community. This review focuses attention on phthalate ester (PAE) extraction procedures, followed by GC-MS analysis, applied to food matrices and developed during the last 15 years (from 2000). In this area, 120 papers have been published, divided according to different sample preparation/extraction methods: liquid–liquid extraction (LLE), solid-phase (micro) extraction (SP(M)E), dispersive liquid–liquid (micro) extraction (DLL(M)E), headspace solid-phase micro-extraction (HS-SPME), microwave extraction, supercritical fluid extraction, ultrasonication extraction, thermal desorption extraction and Soxhlet extraction. Finally, for in-depth information, two important issues, phthalate toxicology and risk assessment and the blank problem in analytical determinations, are discussed.

 Please wait while we load your content...
Please wait while we load your content...