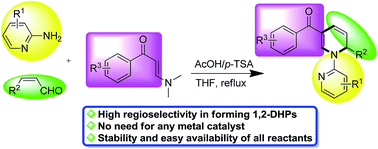
Regioselective three-component reactions of enaminones, 2-aminopyridines and enals for the synthesis of 1,2-dihydropyridines†
Abstract
A three-component synthetic method involving the assembly of enals, N,N-disubstituted enaminones and 2-aminopyridines has been designed, which leads to the facile and regioselective synthesis of 1,2-dihydropyridines (1,2-DHPs) with broad scope and generally good yields in the presence of simple acid catalysts.

 Please wait while we load your content...
Please wait while we load your content...