Theoretical investigation of the mechanism of gold(i)-catalyzed hydrothiolation of alkynes and alkenes with phenthiol†
Abstract
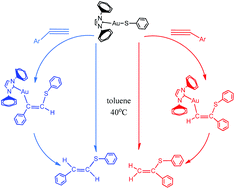
The mechanisms of the gold-catalyzed hydrothiolation of alkynes and alkenes with phenthiol have been investigated using density functional theory calculations done at the B3LYP/6-31G (d,p) (SDD for Au) level of theory. The solvent effect was taken into account by B3LYP/6-311++G (d,p) (SDD for Au) single-point calculations with the integral equation formalism polarizable continuum model (IEFPCM) and solvation model (SMD) in toluene. The calculations indicated that the reactions of the gold-catalyzed hydrothiolation proceed through two competing pathways and lead to Markovnikov-type sulfides or anti-Markovnikov-type products. The process of forming anti-Markovnikov-type products is more favored kinetically with barriers of 21.9 and 23.6 kcal mol−1 for alkyne and olefin versus >27.0 kcal mol−1 for the pathway of forming Markovnikov-type products. The computational results are consistent with the experimental observations of Corma and co-workers. Furthermore, comparing the different metal catalysts of silver and copper for the activity and regioselectivity of the hydrothiolation, the results indicate that the silver catalyst has a relatively high catalytic activity, but the regioselectivity is not satisfactory compared to the gold catalyst and the regioselectivity of copper catalysts is the worst.

 Please wait while we load your content...
Please wait while we load your content...