Beta-lactoglobulin-based encapsulating systems as emerging bioavailability enhancers for nutraceuticals: a review
Zi Teng
 ,
Ruoyang Xu
and
Qin Wang
*
,
Ruoyang Xu
and
Qin Wang
*
Department of Nutrition and Food Science, University of Maryland, 0112 Skinner Building, College Park, MD 20742, USA. E-mail: wangqin@umd.edu; Fax: +1-301-314-3313; Tel: +1-301-405-8421
First published on 8th April 2015
Abstract
In the past few decades, encapsulation has emerged as a promising strategy to enhance the bioavailability of poorly absorbed nutraceuticals. Proteins as natural polymers are generally recognized as safe (GRAS), and they exhibit unique advantages such as natural abundance, amphiphilic nature, satisfactory biodegradability, and desirable functional properties. Beta-lactoglobulin (BLG) is the major component of whey protein and a natural transporter for a number of nutrients. The superior functionality along with marked resistance against peptic digestion enables the preparation of diverse forms of BLG-based encapsulating and delivering vehicles for bioactive compounds. This review article starts with introducing the basic concepts on encapsulation, together with the advantageous properties of BLG with emphasis on the structure–function relation. Afterwards, delivery systems in different forms (simple molecular complexes, nanoparticles, nanoemulsions, and gels) using BLG alone or combining BLG with other polymers are compared systematically with regard to their strengths, weaknesses, and potential applications. Lastly, the challenges and prospective areas of study related to BLG-based delivery systems are discussed.
Ruoyang Xu is a PhD student of Food Nanotechnology in the Department of Nutrition and Food Science at the University of Maryland. She received her Master of Science degree in Food Safety and Technology from Illinois Institute of Technology in Chicago in 2013. Her master's expertise includes food microbiology, food pathogens, molecular microbiology, and foodborne virology. The research in her PhD mainly focuses on nanotechnology, with emphasis on food chemistry, protein chemistry, food polymer science, and materials science. |
1. Introduction
The demands for natural bioactive compounds with health-promoting and disease-preventing benefits have gained much attention recently from the scientific community and food industry. However, the biological efficacies of nutraceuticals are considerably compromised by their low bioavailability, which arises from various factors such as vulnerability to harsh treatments during food processing (heat, oxygen, light, etc.), instability in the gastrointestinal (GI) tract (extreme pH, enzymes, presence of other nutrients), and poor solubility/permeability under changing physiological conditions.1 To enhance the bioavailability of nutraceuticals, various encapsulating and delivery systems have been designed to protect and deliver bioactive compounds to the physiological target.2Various possible benefits can be offered by the encapsulation techniques. The main goals of encapsulation are to (1) protect sensitive or unstable compounds from degradation under adverse conditions, such as exposure to chemicals (oxygen, acid, etc.) and light, and (2) control the bioaccessibility and bioavailability of the encapsulated compounds, and (3) enable target delivery at a particular place within the organism. Encapsulation also provides advantages in converting liquid samples into easily handled powder, masking unpleasant odor or taste of the core material, preserving volatile flavors/aromas, improving stability in final products and during processing, adjusting the properties of active agents, etc.3 By far, numerous encapsulation strategies and systems have been developed to protect polyphenols, herbal extracts, food-fortifying compounds (vitamins, minerals, fish oils, peptides, etc.), and probiotics/microbes(lactobacilli, bifidobacteria) in food systems.4
Among the materials that have been studied as encapsulants, proteins have attracted extensive interest in the past few decades. Proteins are amphiphilic biopolymers which are able to interact sufficiently with both the nutraceuticals and solvents.5 Besides, as naturally occurring polymers, they exhibit lower toxicity and better biodegradability compared to synthetic polymers.6 The desirable functional properties of proteins, including emulsifying and gelling properties,7 together with their flexible conformation, make proteins a versatile template which can be processed into various forms of encapsulating systems suitable for different applications. BLG is a major whey protein in bovine milk. It possesses several unique advantages, such as the possession of natural nutrient binding sites, high water solubility, and resistance against peptic digestion, all of which make it an attractive candidate as a bioavailability enhancer for poorly absorbed nutraceuticals.
This review article is specifically focused on BLG-based encapsulating systems for incorporation and delivery of nutraceuticals. We will start with introducing the basic concepts on encapsulation, together with the structural and functional properties of BLG. Afterwards, different types of BLG-based vehicles such as nanoparticles, emulsions, and BLG–polysaccharide complex systems will be introduced systematically. The advantages and disadvantages of each system will be discussed and explained by the characteristics of BLG. Finally, the challenges and perspective studies associated with BLG-based encapsulating systems will be suggested.
2. Introduction on BLG
BLG is a food-based biopolymer which makes up 60% of whey protein.8 Consisting of 162 amino acids in its sequence, BLG exhibits an average molecular weight of ∼18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 Da and an isoelectric point (pI) of pH 5.1–5.2.9 Several genetic variants occur naturally with modifications on several amino acids residues.10 This protein exists majorly as a dimer at neutral pH, and it dissociates into the monomeric form at pH 3 with the presence of salt.11 The denaturation temperature of BLG is 74 °C at ambient pH and zero ionic strength. This temperature increases to around 80 °C when the pH nears the pI,12 and it can be further elevated in the presence of salts12 and other proteins (e.g., casein).13
400 Da and an isoelectric point (pI) of pH 5.1–5.2.9 Several genetic variants occur naturally with modifications on several amino acids residues.10 This protein exists majorly as a dimer at neutral pH, and it dissociates into the monomeric form at pH 3 with the presence of salt.11 The denaturation temperature of BLG is 74 °C at ambient pH and zero ionic strength. This temperature increases to around 80 °C when the pH nears the pI,12 and it can be further elevated in the presence of salts12 and other proteins (e.g., casein).13
In spite of the extensive studies on the structural and physicochemical properties of BLG, the biological function of this protein remains unsettled. It is widely accepted that BLG belongs to the lipocalin family, which is in responsible for the transport for hydrophobic nutrients.14 Quite a few bioactive molecules have been reported to bind with BLG in previous studies, including retinol,15 vitamin D2,16 fatty acids,17 phenolic compounds,18 and cholesterol.19 At least two binding packets are confirmed in a single BLG molecule, which can bind two different ligands simultaneously.14 The structure and function of the binding sites have been well documented in previous reviews, and an illustration on these sites is given in Fig. 1.16 Associative forces such as hydrogen bonding, hydrophobic interaction, and van der Waal interaction are major contributors to ligand binding. It is arguable, however, if possession of ligand-binding sites guarantees nutrient transport as the major function of BLG, since BLG may be involved in other biological activities which also require such ligand-binding capacity. For example, peptide sequences with angiotensin I-converting enzyme (ACE) inhibitory activity were identified from BLG.20 This finding provides some indirect evidence on the alternative biological roles of this protein.
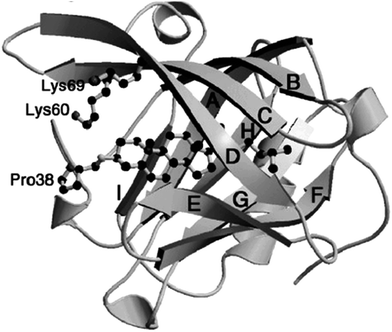 | ||
| Fig. 1 An illustration showing the binding of cholesterol to BLG. The letters A through H designate the eight beta strands in the BLG sequence. Source: ref. 16. | ||
In Section 1, we have discussed the advantage of proteins as effective encapsulants over polysaccharides and synthetic polymers, including flexible structure (which implies the ability to be processed into various forms of encapsulating systems), possession of multiple functional groups (which suggests the easiness for chemical modification), amphiphilic nature (which indicates adequate interaction with entrapped compounds), and desirable biodegradability. Compared to more hydrophobic proteins such as zein and wheat gluten, BLG exhibits superior solubility at a wide range of pH and ionic strengths. On the other hand, since it possesses relatively low content of hydrophobic amino acids (53.4%, molar ratio),21 it may not provide high encapsulation efficiency for hydrophobic bioactive compounds. Complexation with these hydrophobic proteins22 may provide a feasible strategy for achieving better encapsulation efficiency.
Moreover, compared with other common food-derived proteins, BLG possesses two unique properties. The first property lies in its resistance against pepsin,9 the major protease in human's stomach. Three factors are considered to account for such feature. Firstly, pepsin is known to cleave peptide bonds at the hydrophobic patch of protein;23 however, the peptic digestion of BLG is limited by its abundance in charged and polar amino acids. In addition, BLG contains a high content (>55%) of rigid beta-sheet structure (Fig. 1), which reduces its molecular flexibility to some extent and prevents pepsin from approaching and associating with the substrate. Finally, the existence of two disulfide bonds (Cys82–Cys176, and Cys122–Cys135/137 depending on the type of variants) in BLG further stabilizes the protein structure from dissociation.23 On the other hand, BLG can be slowly digested by trypsin in the small intestine. These two digestive properties make BLG an attractive encapsulant for the controlled release of labile nutraceuticals or drugs in the GI tract. Another advantage of BLG is the possession of inherent ligand-binding patches as shown in this section. Such ligand-binding capacity makes BLG an exceptional carrier for nutraceuticals. In the next section, a number of encapsulating systems synthesized from BLG, and their strengths and weaknesses will be compared in details.
3. Key factors for designing encapsulation and delivery systems
A number of factors determine the stability and efficacy of an encapsulation and delivery system. These properties are closely related to the interaction of the matrix with both the nutraceutical and the environment. The physicochemical properties, especially the surface properties of the encapsulant, have a significant impact on these interactions, thus influencing their performances in different physiological processes, as summarized in Table 1.| Properties | Description | Contributing factors |
|---|---|---|
| Loading capacity | Weight (or molar) ratio between the entrapped compound and the encapsulant. Indicates the efficiency of encapsulation | Compound-matrix interaction (electrostatic, hydrophobic, hydrogen bonding. Van der Waals, etc.) |
| Dispersion stability | Stability against precipitation. Contributes to the solubility and absorption of entrapped compounds | Electric charge, hydrophilic groups, and steric hindrance on the surface |
| Controlled release | Release at desired time or locales, or upon exposure to certain stimuli. Improves the efficacy of delivery and minimizes the possible side effect | Suitable polymers or functional groups responsive to certain environmental changes (e.g., pH or enzymes) |
| Mucoadhesion | Adhesion to the mucosa in the gastrointestinal tract. Contributes to the absorption of entrapped compounds | Positive charges on the surface; abundance of hydrogen bond forming groups (e.g., hydroxyl groups) |
| Prolonged circulation | Extended dwelling time in the circulative system. Reduces the loss of bioactive compounds due to opsonization | Steric hindrance or biomimetic polymers on the surface |
| Cellular uptake | Delivery at the cellular level. Ultimate step for delivery | Reduced size of the delivery system; positive surface charge; high surface hydrophobicity; existence of target-specific ligands |
Loading capacity (LC), the weight (or molar) ratio between the encapsulated compound and the matrix, is strongly dependent on the interaction between the entrapped agent and the polymeric matrix.24 Generally, charged compounds tend to attract oppositely charged encapsulants through electrostatic interactions, and hydrophobic chemicals incline to associate with the matrix via hydrophobic interaction.25 Environmental parameters such as pH, ionic strength, and temperature have significant impacts on the type and magnitude of these interactions.26 Therefore, to gain a desirable LC for a bioactive compound of interest in a physiological relevant environment, it is essential to choose an appropriate encapsulant that provides sufficient nutraceutical-matrix association under this specific condition.
Stable dispersion is crucial for the bioavailability enhancement of the incorporated nutraceuticals, and it is largely influenced by the attractive and repulsive interactions among the nutrient carrying vehicles. Attractive interactions include hydrogen bonding, van de Waal interaction, hydrophobic association, and electrostatic attraction. Repulsive interactions, on the other hand, include electrostatic repulsion and steric hindrance. The possession of hydrophobic (e.g., aromatic rings) or hydrophilic groups (e.g., –OH or –NH2) is a major contributor for the hydrophobic interaction or hydrogen bonding, respectively. The surface charge plays a critical role in the type (attractive or repulsive) and magnitude of the electrostatic interaction. This parameter is commonly gauged by zeta potential, which is assessed through electrophoretic mobility measurement. In general, colloidal particles or droplets with zeta potentials above 30 mV or below −30 mV are considered to possess “moderate to good” stability in dispersions,27 due to the significant electrostatic repulsion among them. Highly charged polymers (e.g., soy protein, chitosan) have been utilized as encapsulants to achieve such a level of zeta potential. They are also employed as a second coating layer that improves the dispersion stability when poorly charged materials (e.g., zein) are applied for encapsulation.28,29
The next desirable property termed as controlled release indicates the delivery of entrapped molecular at desired times and/or locations in the human body. Typically, the nutrient-matrix interaction imparts the entrapped compound certain degree of controlled or (more precisely) sustained release. Such property, however, may be easily deprived from many encapsulating systems, which are readily decomposed by the acid and enzymes in the stomach upon oral administration. As a result, the entrapped nutraceuticals may be extensively exposed to the strongly acidic environment in the stomach, leading to considerable degradation. Therefore, a proper encapsulant for nutraceuticals should maintain its integrity and keep the bioactive compound from leaking in the stomach. Upon arrival at the small intestine, the major organ for nutraceutical absorption, the encapsulated compounds should be released in a sustained manner, in order to prevent acute toxicity resulting from a suddenly elevated serum level. Many anionic polysaccharides (e.g., carboxymethyl chitosan) are employed as encapsulants with controlled release properties because of their aggregation in the stomach and degradation in the small intestine.30 Protein such as BLG also possesses such unique digestibility, as will be discussed in details in this review article. For some other applications, the encapsulated compounds are to be delivered intact at specific regions (e.g., colon) in the GI tract. In this case, a proper encapsulant is expected to be indigestible by both stomach and small intestine while responding to a specific stimulus on the target site.
Upon oral administration, most bioactive compounds are absorbed into the systematic circulation in the small intestine. Mucin, a negatively charged extracellular glycoprotein, covers the intestinal epithelia as a gel-like layer and serves as the first barrier for the absorption of nutraceuticals.31 The adhesive properties between the encapsulant and mucin known as mucoadhesion is therefore essential for the bioavailability and efficacy of nutraceuticals.32 Cationic polymers such as chitosan exhibits strong mucoadhesive capacity, which is closely related with its electrostatic attraction with mucin.33 However, it is noteworthy that chitosan with a pKa of ∼6.5 (ref. 34) loses most of its positive charges at the intestinal pH (∼7.0). This fact suggests that other associative interactions such as hydrogen bonding and van der Waal force may also contribute significantly to the mucoadhesion of a polymer.
Following the transport through the small intestine, it is crucial for the delivery vehicle to circulate for a sufficiently long period of time until the bioactive components reach the target tissues or organs. However, many types of vehicles are recognized as invasive substances by the immune system, which leads to rapid opsonization and clearance by the macrophages.35 One common approach to prolonged clearance time is surface modification by PEG, whose long polymeric chain provides the encapsulant with considerable steric hindrance. Other strategies such as modification with CD47 (an integrin-associated protein that acts as a marker of “self” in the blood),36 modulation of mechanical properties, engineering particle morphology, and hitchhiking on red blood cells, have been developed to sustain the circulation as well.37
The final step for the delivery is the uptake by target cells. For bioactives that do not require site-specific delivery, their cellular uptake could be improved by carefully tuning the surface properties of the delivery vehicles. For example, cationic vehicles exhibit higher affinity to most types of cells because they adhere effectively to the negatively charged glycoprotein on cell membrane.38 Delivery vehicles with higher surface hydrophobicity are also believed to permeate the cell membrane more rapidly, thus promoting cellular uptake.39,40 For compounds that have effect on specific sites such as cancer cells, they could be incorporated in a polymeric vehicle conjugated with certain ligands such as folic acid, thus achieving target-specific delivery.41
4. BLG-based encapsulating systems
4.1. Encapsulating systems with BLG as a major functional ingredient
As stated in previous sections, the selection of proper encapsulants and encapsulating techniques is critical for satisfactory incorporation and delivery of the target compound. The advantages of BLG introduced in Section 2 allow the preparation of nutraceutical delivery systems with BLG either as a single functional component or in combination with other encapsulants. The former category of vehicles discussed in this section is relatively easy to synthesize and cost effective. The sizes of these systems are generally smaller than the counterpart containing a second coating material, and the LC is usually higher considering that the application of a second layer adds to the total weight of the encapsulant. Four typical systems are discussed in details in the following paragraphs, and a brief summary on these systems is provided in Table 2.| System | Preparation method | Size | Incorporated compounds | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Molecular complex | Simple mixing and incubation | Several nanometers | Phenols,42–45 folic acid,46,47 and unsaturated fatty acids48 | Simple procedure, no toxic chemicals, resistance against pepsin inherited from BLG, small size contributing to transparency | Low LC; sensitivity to environmental change |
| Nanoparticles | Desolvation, ionic gelation, heat treatment followed by high-pressure homogenization | 50–200 nm | Curcumin,43,49,50 phenols,51,52 fatty acids,48 α-tocopherol53 | Compact structure provides good protection, passive targeted delivery, potential delivery of both lipo- and hydro-philic compounds | Harmful crosslinkers; involvement of organic solvents (for desolvation); low surface charge (for ionic gelation); decomposition in the digestive tract |
| Nanoemulsion | Homogenization | 50–200 nm | β-Carotene,54,55 curcumin56 | Transparent product, sustained release, Satisfactory protection to lipophilic bioactives | Thermodynamically unstable; inability to protect polar compounds; destabilization by dilution, drying, and surfactants in the digestive tract |
| Gel | Organic solvent or ion-induced gelation | Protein network | Theophylline,57 sulfamethoxazole,57 α-tocopherol,1,58 iron59,60 | High LC, sustained release | Large pores indicate poor protection; extensive swelling is sometimes undesired |
Sneharani et al. reported the incorporation of curcumin, a natural phenolic compound, into BLG molecules.43 The chemical stability of curcumin in an aqueous dispersion was improved by 6.7-fold when it was entrapped in BLG. At 25 °C and pH 7.0, curcumin interacted with BLG at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (which corresponded to an LC of ∼2.5%) and exhibited an association constant of 1.01 × 105 M−1. The binding occurred at the central calyx of BLG, as suggested by the author using a molecular modeling study. The author further proposed that higher binding efficacy could be achieved with BLG nanoparticles. Details about the nanoparticle systems will be discussed in the next section. Similar studies have also been reported on BLG-resveratrol42 and BLG–docosahexaenoic acid (DHA) complexes,48 showing that complexation with BLG could significantly improve the chemical stability and solubility of these bioactive compounds.
1 (which corresponded to an LC of ∼2.5%) and exhibited an association constant of 1.01 × 105 M−1. The binding occurred at the central calyx of BLG, as suggested by the author using a molecular modeling study. The author further proposed that higher binding efficacy could be achieved with BLG nanoparticles. Details about the nanoparticle systems will be discussed in the next section. Similar studies have also been reported on BLG-resveratrol42 and BLG–docosahexaenoic acid (DHA) complexes,48 showing that complexation with BLG could significantly improve the chemical stability and solubility of these bioactive compounds.
Liang and Subirade systematically studied the acid and thermal stability of BLG–ligand complexes using the fluorescence quenching technique.61 Different binding sites were found for folic acid (inside the groove between the α-helix and β-barrel) and resveratrol (outer surface). Heating promoted and weakened the affinities of BLG towards resveratrol and α-tocopherol, respectively, while it did not exert any significant influence on the BLG–folic acid complex. Acid treatment resulted in the release of folic acid but did not alter the stability of resveratrol. As for α-tocopherol, acidic environment facilitated the release of the ligand molecules bound on the surface but did not disturb the binding in the internal area. This comprehensive study did not only indicate the potential of BLG-containing molecular complexes as effective delivery systems but also suggested the complexity of BLG–ligand interaction in response to different environmental stimuli.
Although BLG is well known for its resistance against pepsin, few reports on the release of the nutrients bound in BLG molecules are available by far. Pérez et al. suggested that complexation with folic acid did not alter the digestion of BLG in the stomach.62 This finding is reasonable, since the nutrient binding occurs in the native binding sites of BLG and does not require a conformational change. Therefore, a controlled release pattern with minimal release in the stomach is expected with BLG–nutrient complexes. However, further studies need to be carried out to test such hypothesis.
The typical process for preparing nanoparticles with highly soluble proteins such as BLG is commonly referred to as de- or anti-solvation (Fig. 2).6,64 When dissolved in water, the BLG molecules exist as compactly folded “spheres” with their negatively charged groups exposed to the solvent (Phase 1). The addition of an antisolvent (e.g., ethanol) triggers the partial unfolding of the protein, exposing its hydrophobic sites that are originally buried in the region. The surface charge of the protein is also deprived by the antisolvent, the latter of which competes for water molecules with BLG (Phase 2). These processes lead to increased hydrophobic association and reduced electrostatic repulsion, both of which facilitate protein aggregation. As the content of antisolvent increases, aggregation becomes more intense, and nearly spherical particles are formed (Phase 3). Nutraceuticals and/or drugs can be incorporated into the protein dispersion by dissolving the compound into the antisolvent. At this point, the desolvation process can be reversed by adding sufficient water or evaporating the antisolvent, after which the formed particles dissociate readily into individual molecules as the solvent polarity increases. In order to retain the particle integrity, chemical crosslinkers such as glutaraldehyde are introduced. The two aldehyde groups on glutaraldehyde react with two primary amine groups on adjacent lysine residues of the protein, creating a covalent bond that maintains the particle structure (Phase 4). After the removal of antisolvent by evaporation (Phase 5), the nanoparticles retain their morphology and no longer dissociate into individual molecules. Meanwhile, as the solvent becomes more polar during evaporation, the surface charge on the protein recovers, conferring the nanoparticles with desirable stability via electrostatic repulsion. As for the nutraceuticals, they are forced to associate either with adjacent nutraceutical molecules or with the protein matrix as driven by the increase in solvent polarity. As will be discussed later, the protein–nutraceutical interaction can be enhanced by modulating the antisolvent content during evaporation.
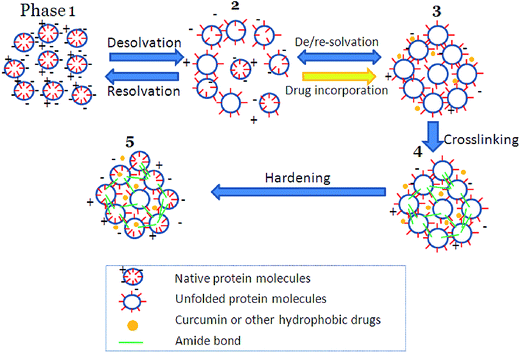 | ||
| Fig. 2 Illustration on the preparation of nanoparticles from globulins. Source: ref. 64. | ||
An alternative method for nanoparticle formation takes advantage of the negative charge on native BLG. Introduction of divalent cations (e.g., Ca2+) or pH adjustment near the pI leads to limited aggregation of BLG into nanoparticles. The drawback of such method is relatively low zeta potentials in the presence of Ca2+ or under acidic environment. To overcome this shortcoming, chemical crosslinkers are added to the dispersion, and the cations or acids are removed after the covalent crosslinking process. The particles with an average diameter of ∼50 nm and desirable dispersion stability can be obtained.65 This procedure shows the potential of encapsulating polar or charged bioactive compounds, which are added to the BLG dispersion during the particle formation step and associate with the formed nanoparticles via electrostatic attraction or hydrogen bonds.
Relkin et al. proposed another effective approach for preparing whey protein concentrate (∼65% BLG) nanoparticles.53 Such procedure involves the dispersion of the protein in water at a relatively high concentration (45 mg mL−1), heating the resultant mixture at 65 °C, and treatment with high speed and high pressure homogenizations. α-Tocopherol as a model compound was successfully incorporated into the protein matrix. Particles with an average size between 150 and 400 nm (dependent on the nutrient/protein weight ratio) were formed, and the zeta potential of −35 to −50 mV indicated desirable stability against precipitation. After 8 weeks of storage, the retention rates of α-tocopherol dispersed in water and encapsulated in the nanoparticles were 32% and 65%, respectively, which demonstrated the significant protection provided by the protein matrix.
Size control is crucial for the preparation of protein nanoparticles. Smaller particle sizes indicate better dispersion stability and larger surface area, both of which are beneficial for the absorption of incorporated nutraceuticals. In addition, particles with an average diameter of 100–600 nm are demonstrated to penetrate the loose blood vessels in the vicinity of tumor tissues and accumulate effectively in tumors, a phenomenon known as enhanced permeation and retention (EPR) effect or passive targeted delivery.66 The size of protein nanoparticles can be determined by several factors including protein concentration, antisolvent content, and type of pretreatments. For instance, higher antisolvent/solvent ratio leads to faster protein unfolding and nucleation, which usually results in the formation of smaller particles with a greater particle number.9,64 Meanwhile, the protein concentration needs to be lowered when higher antisolvent content was chosen, so that the formed nuclei are separated effectively and prevented from excessive aggregation. The selection of antisolvents with lower polarity (e.g., acetone as compared to ethanol) works in a similar way: nucleation is accelerated, and gross protein precipitation should be avoided by choosing lower protein concentration. Thermal treatment at a proper temperature leads to the partial exposure of hydrophobic peptides, thus facilitating the protein agglomeration through hydrophobic interaction. Ko et al. reported the synthesis of sub-100 nm BLG nanoparticles with narrow size distribution.67 The process included preheating the BLG solution at 60 °C to expose the hydrophobic chains, adjusting the pH to 9.0 for better protein dispersion, and adding 80% acetone instead of 80% ethanol to hasten nucleation. The particles sized at 59 ± 5 nm and exhibited a zeta potential below −40 mV at pH 7.
In a recent study, Teng et al. investigated the formation of curcumin-loaded BLG nanoparticles50 with the emphasis on better LC and lower dose of toxic crosslinkers. It was reported that the nutraceutical/matrix interaction plays a determinant role in the LC, and such interaction could be improved by adjusting the antisolvent content to lower values (e.g., 30/70 acetone/water, v/v) after the crosslinking process, followed by slowly increasing the solvent polarity through mild evaporation. High content of antisolvent (e.g., 90/10 acetone/water, v/v), on the other hand, facilitated the dissolvation of the curcumin and weakened its association with the BLG matrix. The highest LC achieved by this study was 11%, which was considerably higher than that achieved by other protein-based single-layer nanoparticles.50 In addition, curcumin as a phenolic compound was revealed to act as a partial crosslinker, which helped reducing the required dose for glutaraldehyde by 50%. Phenolic compounds such as curcumin are able to associate with proteins through extensive hydrogen bonding and π–π interaction, both of which may contribute to the integrity of nanoparticles.
Similar results have also been reported on other phenol-loaded BLG nanoparticles. Shpigelman et al. used thermally denatured BLG to form complex with (−)-epigallocatechin-3-gallate (EGCG), the major catechin in green tea.44 After preheating at 75–85 °C for 20 min, the association constant between the two chemicals increased by 3.5 fold. The as-prepared co-assemblies were smaller than 50 nm, granting the product desirable transparency and enabling their application in clear beverages. These complexes also demonstrated considerable protection to EGCG against oxidative degradation: a 33-fold lower initial degradation rate and a 3.2-fold slower degradation over 8 days were observed for nano-entrapped EGCG compared to the unprotected one. A similar study was conducted by Li et al.68 who reported the synthesis of a clear and stable BLG–EGCG complex solution by preheating at 85 °C at pH 6.4–7.0.
Interestingly, both Ko et al.67 and Teng et al.50 observed rapid decomposition of BLG nanoparticles by pepsin at pH 2, although the individual protein molecules remained undigested. One of the possible reasons for the particle disintegration might be the cleavage of newly formed intermolecular amide bonds created by glutaraldehyde, instead of the breakdown of original peptide backbones. Choosing crosslinkers other than glutaraldehyde may decrease the rate of particle degradation. At pH 5, which corresponds to the moderately acidic gastric environment at the fed state,69 the rate of particle digestion was significantly reduced due to the agglomeration of BLG nanoparticles.
It has been generally recognized that two properties, solubility and surface hydrophobicity, are critical in deciding the emulsifying capacities of proteins.75,76 As introduced in Section 2, BLG possesses exceptional water solubility even near its pI, which favors the stabilization of emulsion. On the other hand, varying values have been reported on the surface hydrophobicity of BLG, using different analytical methods. For instance, the surface hydrophobicity index of BLG determined by 8-anilinonaphthalene-1-sulfonate fluorescent method (S0 ∼ 100, dimensionless, same hereinafter) was more than 20 times lower than that of bovine serum albumin (BSA, S0 > 2000).77 However, using cis-parinaric acid as a fluorescent probe, Kato et al. reported an S0 for BLG (750) that was only twice lower than that of BSA (1400). The latter figure suggests desirable emulsifying capacities for BLG, which has been confirmed by Kato et al.78
Efforts have been put in the past few years to prepare BLG-stabilized nanoemulsions. Qian et al. prepared beta carotene (BC)-loaded nanoemulsions using BLG as an emulsifier.55 The product exhibited an average radius of 78 nm which kept stable within 20 days. In a follow-up study,54 the author demonstrated that BC encapsulated in BLG-stabilized lipid droplets was more stable against chemical degradation than that incorporated within non-ionic surfactant (Tween 20)-coated droplets (Fig. 3). The degradation could be further retarded by adjusting the pH and ionic strength or adding external antioxidants such as EDTA and ascorbic acid. These results demonstrated the potential of BLG-coated nanoemulsion for protecting lipophilic colorants in beverages.
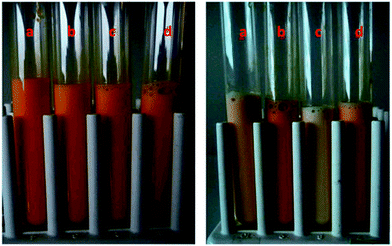 | ||
Fig. 3 Visual appearance of beta-carotene enriched oil-in-water nanoemulsions stabilized by different emulsifiers during storage at 55 °C after 0 days (left) and 15 days (right). Key: (a) BLG no antioxidant; (b) BLG with antioxidant; (c) Tween 20 no antioxidant; (d) Tween 20 with antioxidant. The emulsions contained either no antioxidants (control) or antioxidants (80 μM EDTA + 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm vitamin E acetate). Source: ref. 54. 000 ppm vitamin E acetate). Source: ref. 54. | ||
Ahmed et al. compared BLG-stabilized micro- and nanoemulsions as a delivery system for curcumin.56 The type of oils (short, medium, and long-chain triglycerides, abbreviated as SCT, MCT, and LCT, respectively) played a major role in determining the droplet size: nanoemulsions (droplet size around 200 nm) were formed with LCT, MCT and LCT + SCT, whereas macroemulsion (droplet size around 2 μm) was prepared with SCT alone. The initial digestion rate decreased in the order of SCT > MCT > LCT, while the final extent of digestion decreased as MCT > SCT > LCT. The bioaccessibility of curcumin appraised by a centrifugation method decreased following the sequence of MCT > LCT ≫ SCT. Unexpectedly, the bioaccessibility appeared to be slightly higher in conventional emulsions than in nanoemulsions. The possible reason was that the SCT used for macroemulsion preparation allowed more curcumin molecules (3% curcumin-to-oil weight ratio, same hereinafter) than the MCT (0.8% by weight) or LCT (0.3% by weight) employed for nanoemulsions. As suggested by the authors, the solubilization of curcumin plays a more significant role in determining the bioaccessibility than the droplet size.
The fate of protein-stabilized emulsions in the GI tract is of persisting interest as it determines the bioavailability of the incorporated bioactive compounds. Adsorption of protein molecules to the oil–water interface is often preceded by the partial unfolding of the protein, which might alter the accessibility of digestive enzymes. Such an effect was confirmed by Macierzanka et al., who evaluated the stability of using BLG-stabilized macroemulsions (droplet size 1–10 μm) in simulated digestive fluids without phosphatidylcholine (PC).79,80 In the presence of PC, which displaced the adsorbed BLG at the interface, the resistance of BLG against pepsin was restored. Intriguingly, the digestion of BLG by trypsin and chymotrypsin was also retarded in the presence of PC, which was ascribed by the authors to the formation of PC–BLG complexes. Such phenomenon may lead to altered physicochemical properties of protein-based delivery systems when administrated via oral route.
The methods for preparing protein gels are categorized as thermal and non-thermal processes. Thermal-induced gelation usually involves preheating above the denaturation temperature of a polymer, followed by spontaneous gelation upon cooling. The gelation process usually involves partial or complete unfolding of the protein structure, followed by extensive intermolecular crosslinking through covalent bonds (such as disulfide bonds), hydrogen bonds, hydrophobic interaction, and van der Waal force.81 Chemical crosslinkers such as glutaraldehyde are frequently added, although not required, to harden the gel structure, leading to better mechanical property and decelerated disintegration.82 This method is convenient and provides satisfactory gel strength83 possibly due to the complete denaturation of protein. However, the extensive involvement of heat is unfavorable for the protection of bioactive compounds. Therefore, non-thermal or cold gelation methods have attracted increasing interest for the preparation of novel nutraceutical carriers. Reddy et al. reported a phase separation process for preparing BLG gels in a water–ethanol mixture57 (Fig. 4). The product swelled to 3 to 30 times of its original volume upon hydration, followed by dissolution. A sustained release of two model drugs was observed in 24 h.
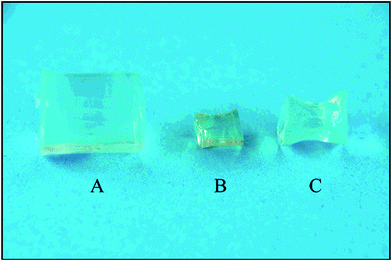 | ||
| Fig. 4 Morphologies of BLG gels formed in 50% (w/v) ethanol–water mixture. (A) freshly hydrated gel (B) dried gel and (C) dried gel rehydrated in PBS. Source: ref. 57. | ||
As another facile gelation method, ion-induced gelation was investigated by several researchers. Electrostatic attraction between proteins and oppositely charged ions (usually multivalent cations such as Ca2+) is the major driving force for gelation. Liang et al.58 prepared α-tocopherol-loaded BLG gels by producing an emulsion coated with BLG followed by the introduction of CaCl2. The resultant emulsion gel demonstrated complete erosion in 6.5 h when incubated in simulated gastric or intestinal fluids. However, when gastric and intestinal digestions were performed successively, the dissolution was significantly slowed down, probably because the partial hydrolysis products of BLG exhibited greater emulsifying properties and stabilized the emulsion gels. Remondetto and others60 prepared BLG gels using Fe2+ as a gelation inducer as well as a bioactive agent. The mechanical properties were improved by increasing BLG concentration but compromised in the presence of excessive Fe2+. The microstructure of the formed gel was dependent on the Fe2+/protein ratio: a homogeneous filamentous network was obtained at a low ratio, whereas more random aggregated particles were present as the proportion of Fe2+ increased.
4.2. Encapsulating systems based on BLG-containing complexes
In addition to the systems described above, an array of complex encapsulants containing BLG and other polymer coatings have been developed to achieve better stability and delivery efficacy. BLG exhibits positive and negative net charges at a pH below or above its pI, respectively, and it also possess abundant hydrophobic and polar amino acid residues. This characteristic allows the complexation between BLG and various polymers (e.g., polysaccharides) to create a bilayer coating via hydrophobic and electrostatic interaction or hydrogen bonding, with or without the presence of additional linkers. The second coating layer generally confers the encapsulated compounds with better protection against chemical and thermal degradations, as well as a more sustained releasing profile. In addition, depending on the nature of the additional polymer, the complex system may exhibit superior performance such as elevated emulsifying capability and better mucoadhesion.Recently, Chen et al.85 reported the encapsulation of a bioactive flavonoid (tangeretin) into zein nanoparticles coated with BLG. The effect of ionic strength, pH and temperature on the stability of the nanoparticles was investigated. The prepared colloidal system was stable at low salt concentrations at pH far from the pI and temperatures below 60 °C. However, particle aggregation occurred at high ionic strength (>100 mmol L−1) or pH near the pI (4.5–5.5) due to decreased electrostatic repulsion. Heating at temperatures over 60 °C in the presence of salt also destabilized the nanoparticles as a result of increased hydrophobic interaction.
4.2.2.1. BLG–polycation complex. Diarrassouba et al.87 incorporated Vitamin D3 successfully in the BLG/lysozyme (Lyso) nanoparticles based on the electrostatic attractions between the two oppositely charged proteins. Particles with a mean diameter of 7.1 ± 2.5 nm were formed at pH 7.5, a BLG/Lyso ratio of 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), and a total protein concentration of 1 mg mL−1. An encapsulation efficiency of 90.8 ± 4.8% was achieved, indicating that the BLG/Lyso complex can be served as a potential delivery vehicle for bioactive compounds. The weight ratio between the loaded vitamin and BLG is estimated to be 2.6% according to the experimental data, assuming that the reported optimal BLG/Lyso ratio was adopted for the vitamin encapsulation study.
1 (w/w), and a total protein concentration of 1 mg mL−1. An encapsulation efficiency of 90.8 ± 4.8% was achieved, indicating that the BLG/Lyso complex can be served as a potential delivery vehicle for bioactive compounds. The weight ratio between the loaded vitamin and BLG is estimated to be 2.6% according to the experimental data, assuming that the reported optimal BLG/Lyso ratio was adopted for the vitamin encapsulation study.Hong et al. reported the production of stable hydrogel particles by thermal treatment (80 °C for 20 min) of BLG (0.5 wt%) and chitosan (0.1 wt%) mixtures at pH 4.5. The biopolymer mixtures formed soluble complexes at pH 4.5 and complex coacervates at pH 5.0–5.5. Preheating at 80 °C and pH 4.5 resulted in the formation of hydrogel particles consisting of a network of aggregated protein and chitosan molecules. These particles exhibited an average diameter of 140 nm and ζ-potential higher than +20 mV. They maintained their initial particle size at the pH range of 3–5 while aggregating at pH > 5 due to a decrease in the electrical charge.88
Ha et al.89 prepared chitosan oligosaccharide (CSO, 20 kDa)/BLG nanoparticles for the encapsulation of quercetin. The synthetic process included mixing the CSO with BLG in 0.1 mol L−1 NaCl solution at pH 4.0–5.5 and ionic crosslinking with sodium tripolyphosphate. Furthermore, the CSO was modified with linoleic acid (LA) to increase the hydrophobicity, leading to an increase in the particle size from 258 to 350 nm, together with a significant improvement in the EE to 55.6%.
4.2.2.2. BLG–polyanion complex. Ron et al. prepared BLG/low methoxyl pectin (LMP) nanoparticles system for the protection and delivery of Vitamin D2.90 The author suggested that the degree of coacervation depended on the pH and pectin content. Larger particles were formed as pectin concentration increased until reaching 0.01% (w/v, same hereinafter) at pH 3.5–4.5, while smaller particles were observed at higher pectin concentrations. The minimal particle size (50–70 nm) was observed at pH 4.25 and 0.05% pectin, at which a clear solution was formed (Fig. 5). Such transparent complex systems may be used for the fortification of hydrophobic nutrients in clear acidic drinks. Similar studies have been conducted on BLG-high methoxyl pectin91 and BLG–carboxymethyl cellulose as well.92
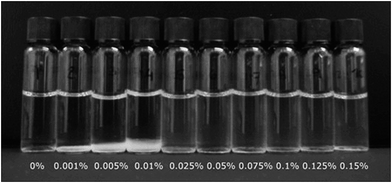 | ||
| Fig. 5 Visual appearance of Vitamin D2-loaded BLG/LMP nanoparticles at different LMP contents. Clear solutions were observed with more than 0.05% LMP. Source: ref. 90. | ||
Guerin et al.93 developed membrane-coated protein–polysaccharide gel beads to protect bifidobacterium, a probiotic bacterium, against gastric acid and bile. The gel was formed with alginate, pectin and whey protein (containing ∼60% BLG). After 1 h of incubation under simulated gastric condition (pH 2.5), the non-encapsulated cells decreased in their population by 4.75 log units, and no live cell was detected after 2 h. On the other hand, the number of encapsulated cells decreased by merely 1 and 2 log units after 1 and 2 h, respectively. After incubation in 2 and 4% bile salt solutions for 1–3 h, the mortality level of bifidobacterium for membrane-free gel beads was 4 to 7 log units compared to less than 2 log units for membrane-coated gel beads. Therefore, the complex gel beads provided marked protection to probiotic bacteria under gastrointestinal conditions.
Gu et al. evaluated the effect of pH and carrageenan type on properties of BLG stabilized oil-in-water emulsions.94 The results indicated that there were electrostatic interactions between carrageenan and BLG in emulsions at pH 3 and 5. As the concentration of carrageenan exceeded a critical level (0.08%, w/v), extensive droplet aggregation and creaming were observed. At pH 6, the average droplet diameter remained relatively small in all emulsions, but only the addition of ι-carrageenan to the emulsions improved their stability compared to conventional emulsions stabilized by a single layered membrane94 (Fig. 6). Similar investigations have been carried out on oil-in water emulsions stabilized by BLG/pectin complexes.93,95
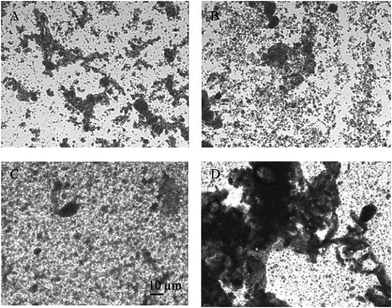 | ||
| Fig. 6 Visual appearance of BLG coated O/W emulsions without carrageenan (A) and with κ (B), ι (C), or λ-carrageenan (D). Source: ref. 94. | ||
4.2.2.3. BLG–neutral biopolymer complex. In addition to the hydrophobic and electrostatic interaction, covalent bonds can be formed between a protein and a polysaccharide through the Maillard reaction. Yi et al.96 encapsulated BC into BLG–dextran conjugated nanoparticles (60–70 nm) by a homogenization–evaporation method. Under simulated gastrointestinal conditions around the pI of BLG (pH 4.0–5.0), nanoparticles formed with BLG as a single encapsulant aggregated extensively, whereas the BLG–dextran particles exhibited significant smaller size. The release of BC in both simulated gastric and intestinal fluid was slower in the complex nanoparticles due to the protection of double coatings. Moreover, the cellular uptake of BC incorporated in BLG and BLG–dextran nanoparticles was improved by about 15 times compared to that of free BC. These results indicated the potential of BLG–dextran conjugated complex nanoparticles as an attractive nutrient carrier.
Lesmes and McClements synthesized BLG–dextran conjugates through Maillard reaction and applied the hybrid polymer to coat lipid droplet for controlling the digestibility of lipid under simulated gastrointestinal conditions.97 The steric hindrance provided by the grafted dextran chain changed the properties of the emulsion, and it also influenced the responsiveness of lipid droplets to pH, pepsin, CaCl2, and bile. Increase in the molecular weight of dextran resulted in enhanced emulsion stability due to enhanced steric hindrance, whereas the lipase digestibility decreased concomitantly.
4.3. Encapsulating systems based on cationic BLG
As discussed in Section 3, the surface charge of a nutraceutical carrier plays a determinant role in the adhesion to mucin and cell membrane, both of which have a significant influence on the bioavailability of the encapsulated compounds. Chitosan is the most widely utilized cationic polymer in food industry due to its natural abundance. However, it is insoluble at neutral to basic pH, which might compromise the claimed mucoadhesion and cellular uptake enhancement in vivo and confine its application to acidic food systems. To combine the strengths of both BLG and cationic polymers, Teng et al. synthesized cationic BLG (CBLG) through a simple amidation reaction using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide as a coupling agent.9 Various cationizers such as polyamines can be employed as cationizers (Fig. 7), conferring the product with different amounts of positive net charges. Nanoparticles with an average size below 100 nm were successfully prepared by acetone desolvation (Fig. 8). The CBLG nanoparticles inherited the desirable solubility and nutraceutical-incorporating capability from native BLG, and it demonstrated significantly elevated mucoadhesion and cellular uptake. In addition, marked resistance against both peptic and tryptic digestion was observed by the CBLG nanoparticles,9,98 probably owing to the steric hindrance provided by the cationizer. Such particle integrity prevented the leakage of encapsulated compounds in the GI tract and ensured the delivery of intact nutraceutical molecules at the cellular level (manuscript submitted for publication).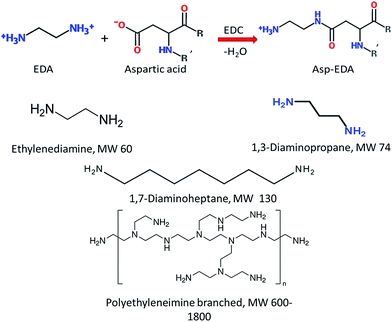 | ||
| Fig. 7 Illustration of the cationization procedure.9 The first row presented the theoretical equation for ethylenediamine-induced cationization. Both Asp and Glu residues were appropriate substrates. The net charge of each residue was altered by +2 (from −1 to +1) upon cationization. The following chemical structures represent the different cationic moieties that may be grafted onto the protein. | ||
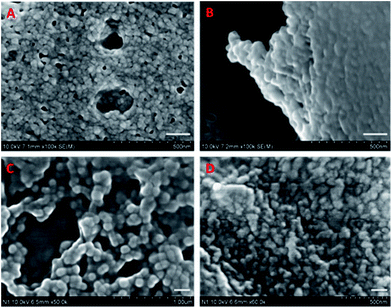 | ||
| Fig. 8 Nanoparticles formed by BLG (A), CBLG using ethylenediamine as a cationizer (B), CBLG using polyethyleneimine as a cationizer (C), and particles in Fig. 6C after evaporation (D). Scale bars represent 100 nm. Source: ref. 98. | ||
In an earlier study by Mattarella et al.,99 cationic BLG derivatives have also been developed through an esterification process. Although that study was focused on functional property improvement rather than encapsulating capacity, it did point out that the surface modified product showed improved emulsifying abilities. This result suggested the potential of cationic BLG in the preparation of nutraceutical-incorporated micro- or nanoemulsions, and it inspires the development of other BLG derivatives with minimal degree of modification for the synthesis of other forms of encapsulating systems.
5. Challenges related with BLG-based encapsulating systems
Despite the various advantages of BLG-based encapsulating systems, several issues must be addressed for their application in food industry. These concerns arise from either the nature of BLG or the preparation process for certain encapsulating systems, as will be discussed below.5.1. Allergenicity
Milk is the most prevalent food allergen worldwide, accounting for the greatest percentage of food allergy in infancy.100 The prevalence of milk allergy in early childhood ranges between 2 and 6%, which decreases markedly in the population at 6 years old and above.101 BLG is the major whey protein in bovine milk without any counterpart in human's milk, and it is known as a chief reason for milk allergy. Several peptide fragments obtained by tryptic digestion have been shown to bind to human IgE102 and IgG,103 thus triggering the allergic reaction.In spite of the apparent risk associated with BLG, the allergenicity of BLG-based nutraceutical-carrying platforms has been rarely studied. Although one can anticipate a conformational change when BLG forms different type of encapsulating and delivering vehicles, it is questionable whether such change is sufficient for altering its allergenic property. In some previous studies on nanoparticles formed by soy protein, another major food protein and known allergen, the secondary structure did not change phenomenally compared to the native protein.63,104 In the case of simple molecular complex, the binding of ligands to BLG is not expected to induce a significant conformational change, since the binding patches already existed in the protein structure before binding takes place. Formation of emulsions and gels may induce a more noticeable change in the protein structure due to the involvement of oil and heat, but no data have been provided to date to demonstrate a reduced allergenicity in these products. In light of these results and speculations, it is of great importance to assess the potential allergenicity arising from BLG.
There are a few approaches that may reduce the risk for BLG-related allergy. Thermal treatments induce reversible and irreversible change in the protein conformation, lowering its capacity to bind with IgE. The IC50 (concentration of BLG to inhibit 50% of the IgE activity) has been reported to increase from 2.03 to 8.45 μg mL−1 when BLG solution was heated at 90 °C for 60 min.105 As discussed before, preheating is applied for preparing BLG nanoparticles with better size uniformity. Therefore, the allergenicity of BLG nanoparticles is anticipated to be lower than that of BLG molecules. Another possible approach to lower allergy risk is chemical modification, especially the conjugation with bulky molecules such as polysaccharides. As a relevant study, Babiker et al. found that glycosylation of soy protein isolate with different polysaccharides through a classical Maillard reaction could remove the allergenicity of soy protein effectively.106 A similar procedure may be rationally applied to BLG. In addition, conjugation with other polymers such as polyethyleneimine may exhibit a comparable effect, which needs further investigation. Lastly, the electrostatic complexation between BLG and polysaccharides such as chitosan may also alter the surface properties of the former, thus reducing its affinity to immunoglobulins. However, the BLG–polysaccharide association must be sustained under different biological conditions with varying pH, ionic strength, and surfactant concentration, in order to exhibit the aforementioned effect.
5.2. Involvement of harmful chemicals
As introduced in previous sections, various chemicals such as organic solvents, crosslinkers, catalysts, and co-surfactants are involved in the preparation of BLG-based nutraceutical carriers. Many of these chemicals possess certain toxicity and elicit considerable health concerns. For example, glutaraldehyde as a common crosslinking agent is toxic to the respiratory and reproductive system, and the genetic toxicity has also been documented.107 Unlike the organic solvents or catalysts which can be removed by evaporation or dialysis, glutaraldehyde is integrated into the nutraceutical carrier by forming chemical bonds, and it may be released in human body when such bonds are cleaved by digestive enzymes. Although BLG nanoparticles have been found to be non-cytotoxic against Caco-2 cells, a colon cancer cell line that is frequently used as a surrogate for intestinal epithelial cells (manuscript submitted for publication), data on the long term or in vivo toxicology of other BLG-based systems are still unavailable. Such facts prompted the pursuit for natural crosslinkers such as genipin108 and microbial transglutaminase (MTGase).109 In spite of the satisfactory performance of these compounds in crosslinking protein molecules and preparing hydrogels,109,110 their application in systems such as nanoparticles has not been reported by far. The compact structure and the short intermolecular distance in the nanoparticles may be the major barrier for enzymes, preventing them from accessing and leaving the reactive sites. Combination of bulky enzymes with flexible substrates (e.g., short peptides or small organic molecules) may help overcoming this difficulty by producing small reactive intermediates as an effective crosslinker. On the other hand, as introduced before, phenols such as EGCG or curcumin show the ability to maintain the particle structure by non-covalent interaction, which may also be employed to synthesize “green” BLG nanoparticles.5.3. Other health concerns
Novel nutraceutical carriers, especially those with a nanoscaled size, have elicited considerable public concern. This is probably because of the lack of knowledge in whether the nanosized nutraceuticals (e.g., lipophilic vitamins that are entrapped in a nanoemulsion droplet) and carriers are metabolized via a similar or distinct pathway in human body compared with conventional microsized nutraceuticals (e.g., lipophilic vitamins that are micro-emulsified by the bile in the small intestine). In addition, it remains unclear whether the existence of a polymeric matrix formed by BLG could facilitate, retard, or alter the normal absorption and metabolism of the encapsulated compound. Model studies are needed to understand the behaviors and fates of both the nutraceuticals and the carriers when administrated to a living body. Establishment of a complete ADME (absorption, absorption, distribution, metabolism, and excretion) profile of each nanodelivery system is helpful for understanding the bioavailability and potential toxicity of the bioactive compound systematically.111 In vitro models such as TIM and SHIME (simulator of the human intestinal microbial ecosystem) may also provide valuable information on the digestion and absorption. The effects of the surface properties and particle/droplet size may be of special interest, since these characteristics govern a wide range of biological interactions.5.4. Concerns regarding industrial production
From a more practical perspective, several issues must be addressed before a protocol for manufacturing BLG-based nutraceutical carriers can be scaled up. Due to the delicate conformation of proteins like BLG, all of the systems described above are susceptible to environmental change. For example, the size and uniformity of the nanoparticles is highly dependent on the organic solvent, pH, temperature, and ionic strength. A slight variation in the microenvironment may lead to either insufficient or excessive aggregation, which poses a daunting challenge to the consistency of product quality. Same challenges also apply to other encapsulation and delivery systems such as emulsions, gels, and molecular complex, which also require careful control over the synthesizing condition.Another concern is the heat treatment involved in traditional food industry such as sterilization and spray drying, both of which will lead to unwanted denaturation of BLG. As introduced in Section 2, the thermal stability of BLG may be improved by adjusting pH, adding salts, or incorporating another polymer as a protectant. Complexation with other polymers as described in Section 4.2 may benefit the thermal stability, although the actual protective effect should be investigated systematically. Application of alternative processing techniques such as lyophilization or non-thermal sterilization is an attractive approach, but they may add to the cost for manufacturing significantly.
6. Conclusions
As illustrated in this review, BLG is a promising material for the preparation of nutraceutical carriers. The desirable properties of BLG include high solubility, natural nutrient-binding capacity and resistance against peptic digestion. These advantages make BLG a versatile component which can be processed into various nutraceutical carrying systems, either by itself or with the aid of other polymers. A broad variety of bioactive compounds with diverse chemical characteristics can be successfully incorporated into the BLG-based systems, thanks to the multiple functional groups and structures that the protein possesses. Further studies are needed not only to address the challenges listed in Section 5, but also to confer BLG-based systems better performances and novel properties. Hereby, we suggest the following areas that may attract researchers' interest in the future:(1) Development of actively targeting delivery vehicles for chemopreventive bioactives. As discussed in Section 3, ligands such as folic acid when conjugated on BLG may provide enhanced delivery of incorporated compounds to cancer cells, owing to its affinity to the folate receptor protein abundant on the surface of tumor cells.112 Proteins such as CD47 (ref. 37) may confer the BLG-based vehicles with “stealth” properties, allowing it to release beneficial compounds for a prolonged period in the circulation system. These “smart” delivery vehicles may be of great interest in contemporary food and pharmaceutical industries.
(2) Sensory properties are another important factor affecting consumer acceptance on the BLG-based nutraceutical carriers. It is of interest to find out whether BLG, a protein giving off a taste of whey, can mask the unpleasant flavor of certain nutraceuticals such as fatty acids or phenols. The effect of particle size on the taste and mouth feel of the product is another topic of interest. As proposed by Velikov et al., particles whose size falls in the range of 100–1000 nm may deliver a satisfying combination of taste and mouth feel. Smaller delivery systems (such as molecular complexes) give off strong and unpleasant flavor probably due to the rapid diffusion, while larger delivery vehicles (such as microparticles) may increase the sandiness or creaming of the product.113 Lastly, the influence of processing (e.g., thermal treatment) on the flavor may also be assessed.
(3) The application of BLG-based carriers in the areas related to the food industry but different from nutraceutical delivery may also be pursued. For instance, BLG with suitable surface modification might serve as a potential carrier for pesticides or antimicrobial agents, providing satisfactory solubility, stability, and cell penetrating efficacy to the incorporated compounds. As an alternative field of application, the unique properties BLG may inspire the synthesis of biomimetic materials, e.g., hybrid films or metallic nanoparticles with a BLG coating that provides desirable ligand-binding capacity or controllable digestion profiles.
References
- L. Chen, G. E. Remondetto and M. Subirade, Food protein-based materials as nutraceutical delivery systems, Trends Food Sci. Technol., 2006, 17, 272–283 CrossRef CAS PubMed.
- B. F. Gibbs, S. Kermasha, I. Alli and C. N. Mulligan, Encapsulation in the food industry: a review, Int. J. Food Sci. Nutr., 1999, 50, 213–224 CrossRef CAS.
- N. Zuidam and E. Shimoni, Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them, in Encapsulation Technologies for Active Food Ingredients and Food Processing, ed. N. J. Zuidam and V. Nedovic, Springer New York, 2010, pp. 3–29 Search PubMed.
- V. Nedovic, A. Kalusevic, V. Manojlovic, S. Levic and B. Bugarski, An overview of encapsulation technologies for food applications, Procedia Food Sci., 2011, 1, 1806–1815 CrossRef CAS PubMed.
- J. J. Marty, R. C. Oppenheim and P. Speiser, Nanoparticles – New Colloidal Drug Delivery System, Pharm. Acta Helv., 1978, 53, 17–23 CAS.
- C. Weber, C. Coester, J. Kreuter and K. Langer, Desolvation process and surface characterisation of protein nanoparticles, Int. J. Pharm., 2000, 194, 91–102 CrossRef CAS.
- L. G. Phillips and S. L. Taylor, Structure–Function Properties of Food Proteins, Food Science and Technology, 1994, pp. 107–109 Search PubMed.
- O. G. Jones, S. Handschin, J. Adamcik, L. Harnau, S. Bolisetty and R. Mezzenga, Complexation of Beta-Lactoglobulin Fibrils and Sulfated Polysaccharides, Biomacromolecules, 2011, 12, 3056–3065 CrossRef CAS PubMed.
- Z. Teng, Y. Li, Y. Luo, B. Zhang and Q. Wang, Cationic β-Lactoglobulin Nanoparticles as a Bioavailability Enhancer: Protein Characterization and Particle Formation, Biomacromolecules, 2013, 14, 2848–2856 CrossRef CAS PubMed.
- M. A. Meza-Nieto, B. Vallejo-Cordoba, A. F. González-Córdova, L. Félix and F. M. Goycoolea, Effect of β-Lactoglobulin A and B Whey Protein Variants on the Rennet-Induced Gelation of Skim Milk Gels in a Model Reconstituted Skim Milk System, J. Dairy Sci., 2007, 90, 582–593 CrossRef CAS.
- K. Sakurai, M. Oobatake and Y. Goto, Salt-dependent monomer-dimer equilibrium of bovine beta-lactoglobulin at pH 3, Protein Sci., 2001, 10, 2325–2335 CrossRef CAS PubMed.
- I. J. Haug, H. M. Skar, G. E. Vegarud, T. Langsrud and K. I. Draget, Electrostatic effects on β-lactoglobulin transitions during heat denaturation as studied by differential scanning calorimetry, Food Hydrocolloids, 2009, 23, 2287–2293 CrossRef CAS PubMed.
- Z. Saadati and A. Razzaghi, Comparative Analysis of Chemical and Thermal Denatured β-Lactoglobulin AB in the Presence of Casein, Advanced Studies in Biology, 2012, 4, 255–264, http://www.m-hikari.com/asb/ Search PubMed.
- G. Kontopidis, C. Holt and L. Sawyer, The ligand-binding site of bovine β-lactoglobulin: evidence for a function?, J. Mol. Biol., 2002, 318, 1043–1055 CrossRef CAS.
- Y. Cho, C. A. Batt and L. Sawyer, Probing the retinol-binding site of bovine beta-lactoglobulin, J. Biol. Chem., 1994, 269, 11102–11107 CAS.
- G. Kontopidis, C. Holt and L. Sawyer, Invited Review: β-Lactoglobulin: Binding Properties, Structure, and Function, J. Dairy Sci., 2004, 87, 785–796 CrossRef CAS.
- M. Narayan and L. J. Berliner, Mapping fatty acid binding to β-lactoglobulin: Ligand binding is restricted by modification of Cys 121, Protein Sci., 1998, 7, 150–157 CrossRef CAS PubMed.
- L. H. Riihimaki, M. J. Vainio, J. M. S. Heikura, K. H. Valkonen, V. T. Virtanen and P. M. Vuorela, Binding of phenolic compounds and their derivatives to bovine and reindeer β-lactoglobulin, J. Agric. Food Chem., 2008, 56, 7721–7729 CrossRef CAS PubMed.
- Q. Wang, J. C. Allen and H. E. Swaisgood, Binding of Vitamin D and Cholesterol to β-Lactoglobulin, J. Dairy Sci., 1997, 80, 1054–1059 CrossRef CAS.
- A. Pihlanto-Leppälä, Bioactive peptides derived from bovine whey proteins: opioid and ace-inhibitory peptides, Trends Food Sci. Technol., 2000, 11, 347–356 CrossRef.
- L. J. Alexander, G. Hayes, M. J. Pearse, C. W. Beattie, A. F. Stewart, I. M. Willis and A. G. Mackinlay, Complete sequence of the bovine beta-lactoglobulin cDNA, Nucleic Acids Res., 1989, 17, 6739 CrossRef CAS PubMed.
- J. Chen, J. Zheng, D. J. McClements and H. Xiao, Tangeretin-loaded protein nanoparticles fabricated from zein/β-lactoglobulin: Preparation, characterization, and functional performance, Food Chem., 2014, 158, 466–472 CrossRef CAS PubMed.
- I. M. Reddy, N. K. D. Kella and J. E. Kinsella, Structural and Conformational Basis of the Resistance of Beta-Lactoglobulin to Peptic and Chymotryptic Digestion, J. Agric. Food Chem., 1988, 36, 737–741 CrossRef CAS.
- M. Jahanshahi and Z. Babaei, Protein nanoparticle: A unique system as drug delivery vehicles, Afr. J. Biotechnol., 2008, 7, 4926–4934 CAS.
- Y. Kawashima, Nanoparticulate systems for improved drug delivery, Adv. Drug Delivery Rev., 2001, 47, 1–2 CrossRef CAS.
- R. Matsuno and S. Adachi, Lipid encapsulation technology-techniques and applications to food, Trends Food Sci. Technol., 1993, 4, 256–261 CrossRef CAS.
- M. Larsson, A. Hill, J. Duffy and M. I. N. Ab, Annu. Trans. - Nord. Rheol. Soc., 2012, 20, 209–214 CAS.
- Y. Luo, Z. Teng and Q. Wang, Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3, J. Agric. Food Chem., 2012, 60, 836–843 CrossRef CAS PubMed.
- Y. Luo, B. Zhang, M. Whent, L. L. Yu and Q. Wang, Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study, Colloids Surf., B, 2011, 85, 145–152 CrossRef CAS PubMed.
- Y. Luo, Z. Teng and Q. Wang, Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3, J. Agric. Food Chem., 2012, 836–843 CrossRef CAS PubMed.
- R. Bansil and B. S. Turner, Mucin structure, aggregation, physiological functions and biomedical applications, Curr. Opin. Colloid Interface Sci., 2006, 11, 164–170 CrossRef CAS PubMed.
- J. Thongborisute and H. Takeuchi, Evaluation of mucoadhesiveness of polymers by BIACORE method and mucin-particle method, Int. J. Pharm., 2008, 354, 204–209 CrossRef CAS PubMed.
- P. M. Claesson and B. W. Ninham, pH-dependent interactions between adsorbed chitosan layers, Langmuir, 1992, 8, 1406–1412 CrossRef CAS.
- A. El-Kamel, M. Sokar, V. Naggar and S. Al Gamal, Chitosan and sodium alginate-based bioadhesive vaginal tablets, AAPS PharmSci, 2002, 4, 224–230 CrossRef PubMed.
- C. Delgado, G. E. Francis and D. Fisher, The Uses and Properties of Peg-Linked Proteins, Crit. Rev. Ther. Drug Carrier Syst., 1992, 9, 249–304 CAS.
- P.-A. Oldenborg, A. Zheleznyak, Y.-F. Fang, C. F. Lagenaur, H. D. Gresham and F. P. Lindberg, Role of CD47 as a Marker of Self on Red Blood Cells, Science, 2000, 288, 2051–2054 CrossRef CAS.
- R. J. Kok, F. Grijpstra, K. H. Nederhoed and F. Moolenaar, Renal drug delivery with low-molecular-weight proteins: The effect of charge modifications on the body distribution of drug-lysozyme conjugates, Drug Delivery, 1999, 6, 1–8 CrossRef CAS.
- S. Blau, T. T. Jubeh, S. M. Haupt and A. Rubinstein, Drug targeting by surface cationization, Crit. Rev. Ther. Drug Carrier Syst., 2000, 17, 425–465 CrossRef CAS.
- Y.-B. Lim, E. Lee and M. Lee, Cell Penetrating Peptide-Coated Nanoribbons for Intracellular Nanocarriers, Angew. Chem., 2007, 119, 3545–3548 CrossRef PubMed.
- M. Lindgren, M. Hällbrink, A. Prochiantz and Ü. Langel, Cell-penetrating peptides, Trends Pharmacol. Sci., 2000, 21, 99–103 CrossRef CAS.
- J. Sudimack and R. J. Lee, Targeted drug delivery via the folate receptor, Adv. Drug Delivery Rev., 2000, 41, 147–162 CrossRef CAS.
- L. Liang, H. A. Tajmir-Riahi and M. Subirade, Interaction of β-Lactoglobulin with Resveratrol and its Biological Implications, Biomacromolecules, 2007, 9, 50–56 CrossRef PubMed.
- A. H. Sneharani, J. V. Karakkat, S. A. Singh and A. G. A. Rao, Interaction of Curcumin with β-Lactoglobulin-Stability, Spectroscopic Analysis, and Molecular Modeling of the Complex, J. Agric. Food Chem., 2010, 58, 11130–11139 CrossRef CAS PubMed.
- A. Shpigelman, G. Israeli and Y. D. Livney, Thermally-induced protein–polyphenol co-assemblies: beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG, Food Hydrocolloids, 2010, 24, 735–743 CrossRef CAS PubMed.
- S. L. Maux, L. Giblin, T. Croguennec, S. Bouhallab and A. Brodkorb, β-Lactoglobulin as a Molecular Carrier of Linoleate: Characterization and Effects on Intestinal Epithelial Cells in Vitro, J. Agric. Food Chem., 2012, 60, 9476–9483 CrossRef PubMed.
- L. Liang, J. Zhang, P. Zhou and M. Subirade, Protective effect of ligand-binding proteins against folic acid loss due to photodecomposition, Food Chem., 2013, 141, 754–761 CrossRef CAS PubMed.
- L. Liang and M. Subirade, β-Lactoglobulin/folic acid complexes: formation, characterization, and biological implication, J. Phys. Chem. B, 2010, 114, 6707–6712 CrossRef CAS PubMed.
- P. Zimet and Y. D. Livney, Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids, Food Hydrocolloids, 2009, 23, 1120–1126 CrossRef CAS PubMed.
- S. L. Maux, S. Bouhallab, L. Giblin, A. Brodkorb and T. Croguennec, Complexes between linoleate and native or aggregated β-lactoglobulin: Interaction parameters and in vitro cytotoxic effect, Food Chem., 2013, 141, 2305–2313 CrossRef PubMed.
- Z. Teng, Y. Li and Q. Wang, Insight into Curcumin-Loaded β-Lactoglobulin Nanoparticles: Incorporation, Particle Disintegration, and Releasing Profiles, J. Agric. Food Chem., 2014, 62, 8837–8847 CrossRef CAS PubMed.
- Z. Li, J.-H. Ha, T. Zou and L. Gu, Fabrication of Coated Bovine Serum Albumin (BSA)-Epigallocatechin gallate (EGCG) Nanoparticles and Their Transport across Monolayers of Human Intestinal Epithelial Caco-2 Cells, Food Funct., 2014, 1278–1285 CAS.
- A. Shpigelman, Y. Cohen and Y. D. Livney, Thermally-induced beta-lactoglobulin-EGCG nanovehicles: Loading, stability, sensory and digestive-release study, Food Hydrocolloids, 2012, 29, 57–67 CrossRef CAS PubMed.
- P. Relkin and R. Shukat, Food protein aggregates as vitamin-matrix carriers: Impact of processing conditions, Food Chem., 2012, 134, 2141–2148 CrossRef CAS PubMed.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Inhibition of β-carotene degradation in oil-in-water nanoemulsions: Influence of oil-soluble and water-soluble antioxidants, Food Chem., 2012, 135, 1036–1043 CrossRef CAS PubMed.
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type, Food Chem., 2011, 132, 1221–1229 CrossRef PubMed.
- K. Ahmed, Y. Li, D. J. McClements and H. Xiao, Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties, Food Chem., 2012, 132, 799–807 CrossRef CAS PubMed.
- T. T. Reddy, L. Lavenant, J. Lefebvre and D. Renard, Swelling Behavior and Controlled Release of Theophylline and Sulfamethoxazole Drugs in β-Lactoglobulin Protein Gels Obtained by Phase Separation in Water–Ethanol Mixture, Biomacromolecules, 2005, 7, 323–330 CrossRef PubMed.
- L. Liang, V. Leung Sok Line, G. E. Remondetto and M. Subirade, In vitro release of α-tocopherol from emulsion-loaded β-lactoglobulin gels, Int. Dairy J., 2010, 20, 176–181 CrossRef CAS PubMed.
- A. H. Martin and G. A. H. de Jong, Enhancing the in vitro Fe2+ bio-accessibility using ascorbate and cold-set whey protein gel particles, Dairy Sci. Technol., 2012, 92, 133–149 CrossRef CAS PubMed.
- G. E. Remondetto, P. Paquin and M. Subirade, Cold Gelation of β-lactoglobulin in the Presence of Iron, J. Food Sci., 2002, 67, 586–595 CrossRef CAS PubMed.
- L. Liang and M. Subirade, Study of the acid and thermal stability of β-lactoglobulin–ligand complexes using fluorescence quenching, Food Chem., 2012, 132, 2023–2029 CrossRef CAS PubMed.
- O. E. Pérez, T. David-Birman, E. Kesselman, S. Levi-Tal and U. Lesmes, Milk protein–vitamin interactions: Formation of beta-lactoglobulin/folic acid nano-complexes and their impact on in vitro gastro-duodenal proteolysis, Food Hydrocolloids, 2014, 38, 40–47 CrossRef PubMed.
- Z. Teng, Y. Luo and Q. Wang, Carboxymethyl Chitosan-Soy Protein Complex Nanoparticles for the Encapsulation and Controlled Release of Vitamin D3, Food Chem., 2013, 141, 524–532 CrossRef CAS PubMed.
- Z. Teng, Y. C. Luo and Q. Wang, Nanoparticles Synthesized from Soy Protein: Preparation, Characterization, and Application for Nutraceutical Encapsulation, J. Agric. Food Chem., 2012, 60, 2712–2720 CrossRef CAS PubMed.
- H. J. Giroux, J. Houde and M. Britten, Preparation of nanoparticles from denatured whey protein by pH-cycling treatment, Food Hydrocolloids, 2009, 24, 341–346 CrossRef PubMed.
- C. C. Fleischer and C. K. Payne, Nanoparticle surface charge mediates the cellular receptors used by protein–nanoparticle complexes, J. Phys. Chem. B, 2012, 116, 8901–8907 CrossRef CAS PubMed.
- S. Ko and S. Gunasekaran, Preparation of sub-100-nm beta-lactoglobulin (BLG) nanoparticles, J. Microencapsulation, 2006, 23, 887–898 CrossRef CAS PubMed.
- B. Li, W. Du, J. Jin and Q. Du, Preservation of (−)-Epigallocatechin-3-gallate Antioxidant Properties Loaded in Heat Treated β-Lactoglobulin Nanoparticles, J. Agric. Food Chem., 2012, 60, 3477–3484 CrossRef CAS PubMed.
- N. Fotaki and M. Vertzoni, Biorelevant Dissolution Methods and Their Applications in In Vitro–In Vivo Correlations for Oral Formulations, Open Drug Delivery J., 2010, 4, 2–13 CrossRef CAS.
- Y. Li, Z. Teng, P. Chen, Y. Song, Y. Luo and Q. Wang, Enhancement of aqueous stability of allyl isothiocyanate using nanoemulsions prepared by an emulsion inversion point method, J. Colloid Interface Sci., 2015, 438, 130–137 CrossRef CAS PubMed.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Formation and stability of nano-emulsions, Adv. Colloid Interface Sci., 2004, 108, 303–318 CrossRef PubMed.
- D. J. McClements, Nanoemulsions versus microemulsions: terminology, differences, and similarities, Soft Matter, 2012, 8, 1719–1729 RSC.
- A. A. Date, N. Desai, R. Dixit and M. Nagarsenker, Self-nanoemulsifying drug delivery systems: formulation insights, applications and advances, Nanomedicine, 2010, 5, 1595–1616 CrossRef CAS PubMed.
- Y. Li, Z. Teng, P. Chen, Y. Song, Y. Luo and Q. Wang, Enhancement of aqueous stability of allyl isothiocyanate using nanoemulsions prepared by an emulsion inversion point method, J. Colloid Interface Sci., 2015, 438, 130–137 CrossRef CAS PubMed.
- L. P. Voutsinas, E. Cheung and S. Nakai, Relationships of hydrophobicity to emulsifying properties of heat denatured proteins, J. Food Sci., 1983, 48, 26–32 CrossRef CAS PubMed.
- J. R. Wagner and J. Gueguen, Surface functional properties of native, acid-treated, and reduced soy glycinin. 2. Emulsifying properties, J. Agric. Food Chem., 1999, 47, 2181–2187 CrossRef CAS PubMed.
- N. Alizadeh-Pasdar and E. C. Y. Li-Chan, Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes, J. Agric. Food Chem., 2000, 48, 328–334 CrossRef CAS PubMed.
- A. Kato and S. Nakai, Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins, Biochim. Biophys. Acta, Protein Struct., 1980, 624, 13–20 CrossRef CAS.
- A. Macierzanka, A. I. Sancho, E. N. C. Mills, N. M. Rigby and A. R. Mackie, Emulsification alters simulated gastrointestinal proteolysis of β-casein and β-lactoglobulin, Soft Matter, 2009, 5, 538–550 RSC.
- A. Mackie and A. Macierzanka, Colloidal aspects of protein digestion, Curr. Opin. Colloid Interface Sci., 2009, 15, 102–108 CrossRef PubMed.
- A. Totosaus, J. G. Montejano, J. A. Salazar and I. Guerrero, A review of physical and chemical protein gel induction, Int. J. Food Sci. Technol., 2002, 37, 589–601 CrossRef CAS.
- Y. Luo, Z. Teng, X. Wang and Q. Wang, Development of carboxymethyl chitosan hydrogel beads in alcohol–aqueous binary solvent for nutrient delivery applications, Food Hydrocolloids, 2013, 31, 332–339 CrossRef CAS PubMed.
- S. Jeyarajah and J. C. Allen, Calcium binding and salt-induced structural changes of native and preheated beta-lactoglobulin, J. Agric. Food Chem., 1994, 42, 80–85 CrossRef CAS.
- Y. Luo, T. Wang, Z. Teng, P. Chen, J. Sun and Q. Wang, Encapsulation of indole-3-carbinol and 3,3′-diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability, Food Chem., 2013, 139, 224–230 CrossRef CAS PubMed.
- J. Chen, J. Zheng, D. J. McClements and H. Xiao, Tangeretin-loaded protein nanoparticles fabricated from zein/β-lactoglobulin: Preparation, characterization, and functional performance, Food Chem., 2014, 158, 466–472 CrossRef CAS PubMed.
- O. G. Jones and D. J. McClements, Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein–polysaccharide complexes, Adv. Colloid Interface Sci., 2011, 167, 49–62 CrossRef CAS PubMed.
- F. Diarrassouba, G. Remondetto, G. Garrait, P. Alvarez, E. Beyssac and M. Subirade, Self-assembly of β-lactoglobulin and egg white lysozyme as a potential carrier for nutraceuticals, Food Chem., 2015, 173, 203–209 CrossRef CAS PubMed.
- Y.-H. Hong and D. J. McClements, Formation of hydrogel particles by thermal treatment of β-lactoglobulin–chitosan complexes, J. Agric. Food Chem., 2007, 55, 5653–5660 CrossRef CAS PubMed.
- H.-K. Ha, J. W. Kim, M.-R. Lee and W.-J. Lee, Formation and characterization of quercetin-loaded chitosan oligosaccharide/β-lactoglobulin nanoparticle, Food Res. Int., 2013, 52, 82–90 CrossRef CAS PubMed.
- N. Ron, P. Zimet, J. Bargarum and Y. D. Livney, Beta-lactoglobulin–polysaccharide complexes as nanovehicles for hydrophobic nutraceuticals in non-fat foods and clear beverages, Int. Dairy J., 2010, 20, 686–693 CrossRef CAS PubMed.
- O. G. Jones, E. A. Decker and D. J. McClements, Comparison of protein–polysaccharide nanoparticle fabrication methods: Impact of biopolymer complexation before or after particle formation, J. Colloid Interface Sci., 2010, 344, 21–29 CrossRef CAS PubMed.
- L. Carpineti, M. J. Martinez, A. M. Pilosof and O. E. Pérez, β-Lactoglobulin–carboxymethylcellulose core–shell microparticles: Construction, characterization and isolation, J. Food Eng., 2014, 131, 65–74 CrossRef CAS PubMed.
- D. Guérin, J.-C. Vuillemard and M. Subirade, Protection of bifidobacteria encapsulated in polysaccharide–protein gel beads against gastric juice and bile, J. Food Prot., 2003, 66, 2076–2084 Search PubMed.
- Y. S. Gu, E. A. Decker and D. J. McClements, Influence of pH and carrageenan type on properties of β-lactoglobulin stabilized oil-in-water emulsions, Food Hydrocolloids, 2005, 19, 83–91 CrossRef CAS PubMed.
- D. Guzey, H. Kim and D. J. McClements, Factors influencing the production of o/w emulsions stabilized by β-lactoglobulin–pectin membranes, Food Hydrocolloids, 2004, 18, 967–975 CrossRef CAS PubMed.
- J. Yi, T. I. Lam, W. Yokoyama, L. W. Cheng and F. Zhong, Controlled Release of β-Carotene in β-Lactoglobulin–Dextran-Conjugated Nanoparticles' in Vitro Digestion and Transport with Caco-2 Monolayers, J. Agric. Food Chem., 2014, 62, 8900–8907 CrossRef CAS PubMed.
- U. Lesmes and D. J. McClements, Controlling lipid digestibility: Response of lipid droplets coated by β-lactoglobulin–dextran Maillard conjugates to simulated gastrointestinal conditions, Food Hydrocolloids, 2012, 26, 221–230 CrossRef CAS PubMed.
- Z. Teng, Y. Li, Y. Niu, Y. Xu, L. Yu and Q. Wang, Cationic β-lactoglobulin nanoparticles as a bioavailability enhancer: Comparison between ethylenediamine and polyethyleneimine as cationizers, Food Chem., 2014, 159, 333–342 CrossRef CAS PubMed.
- N. L. Mattarella and T. Richardson, Physicochemical and functional properties of positively charged derivatives of bovine beta-lactoglobulin, J. Agric. Food Chem., 1983, 31, 972–978 CrossRef CAS.
- R. A. Wood, The natural history of food allergy, Pediatrics, 2003, 111, 1631–1637 Search PubMed.
- B.-M. Exl and R. Fritsché, Cow's milk protein allergy and possible means for its prevention, Nutrition, 2001, 17, 642–651 CrossRef CAS.
- I. Selo, G. Clement, H. Bernard, J. Chatel, C. Creminon, G. Peltre and J. Wal, Allergy to bovine beta-lactoglobulin: specificity of human IgE to tryptic peptides, Clin. Exp. Allergy, 1999, 29, 1055–1063 CrossRef CAS.
- K. M. Jarvinen, P. Chatchatee, L. Bardina, K. Beyer and H. A. Sampson, IgE and IgG binding epitopes on alpha-lactalbumin and beta-lactoglobulin in cow's milk allergy, Int. Arch. Allergy Immunol., 2001, 126, 111–118 CrossRef CAS PubMed.
- J. Zhang, L. Liang, Z. Tian, L. Chen and M. Subirade, Preparation and in vitro evaluation of calcium-induced soy protein isolate nanoparticles and their formation mechanism study, Food Chem., 2012, 133, 390–399 CrossRef CAS PubMed.
- B.-M. Ehn, B. Ekstrand, U. Bengtsson and S. Ahlstedt, Modification of IgE Binding during Heat Processing of the Cow's Milk Allergen β-Lactoglobulin, J. Agric. Food Chem., 2004, 52, 1398–1403 CrossRef CAS PubMed.
- E. F. E. Babiker, A. Hiroyuki, N. Matsudomi, H. Iwata, T. Ogawa, N. Bando and A. Kato, Effect of polysaccharide conjugation or transglutaminase treatment on the allergenicity and functional properties of soy protein, J. Agric. Food Chem., 1998, 46, 866–871 CrossRef CAS.
- NTP Technical Report on Toxicity Studies of Glutaraldehyde Administered by Inhalation to F344/N Rats and B6C3F 1 Mice. NIH Publication 93–3348, dated March 1993. US Department of Health and Human Services. In National Toxicology Program: 1993.
- S.-C. Chen, Y.-C. Wu, F.-L. Mi, Y.-H. Lin, L.-C. Yu and H.-W. Sung, A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery, J. Controlled Release, 2004, 96, 285–300 CrossRef CAS PubMed.
- S. Y. Tanimoto and J. E. Kinsella, Enzymic modification of proteins: effects of transglutaminase cross-linking on some physical properties of beta-lactoglobulin, J. Agric. Food Chem., 1988, 36, 281–285 CrossRef CAS.
- M. Fargemand, B. S. Murray and E. Dickinson, Cross-linking of milk proteins with transglutaminase at the oil–water interface, J. Agric. Food Chem., 1997, 45, 2514–2519 CrossRef.
- T. Borel and C. M. Sabliov, Nanodelivery of bioactive components for food applications: types of delivery systems, properties, and their effect on ADME profiles and toxicity of nanoparticles, Annu. Rev. Food Sci. Technol., 2014, 5, 197–213 CrossRef CAS PubMed.
- Z. Teng, Y. Luo, T. Wang, B. Zhang and Q. Wang, Development and Application of Nanoparticles Synthesized with Folic Acid Conjugated Soy Protein, J. Agric. Food Chem., 2013, 61, 2556–2564 CrossRef CAS PubMed.
- K. P. Velikov and E. Pelan, Colloidal delivery systems for micronutrients and nutraceuticals, Soft Matter, 2008, 4, 1964–1980 RSC.
| This journal is © The Royal Society of Chemistry 2015 |


