Calf thymus DNA characterization and its adsorption on different silica surfaces
Senem Yetgin* and
Devrim Balkose
Department of Chemical Engineering, Izmir Institute of Technology, 35430 Gulbahce Urla, Izmir, Turkey. E-mail: devrimbalkose@iyte.edu.tr; senemyetgin@gmail.com
First published on 17th June 2015
Abstract
DNA adsorption is the initial stage of gene therapy for drug delivery systems and solid phase extraction methods of DNA purification. High pore volume and high adsorption capacity are simple requirements not only for producing ‘smart’ drug delivery systems but also the development of purification kits. Silica is the most used material for this purpose. The present study aimed at elucidating the calf thymus DNA biosorption process by the characterization of calf thymus DNA and silica to increase the efficiency of the currently used silica material. Mesoporous silica has long been used for DNA adsorption and silica aerogel is the new adsorbent investigated in the present study. When DNA solution was freeze dried on a silica wafer, self-assembled super helices formed as shown by atomic microscopy (AFM). Thus DNA existed not as single molecules but as large sized agglomerates in water. Thus it could be adsorbed in the macropores and on the external surface of adsorbents. Adsorption of calf thymus DNA to a silica aerogel, a mesoporous silica gel and a silica wafer was investigated in the present study. Silica aerogel was synthesized from TEOS by a supercritical ethanol drying process. The DNA adsorption capacity of the silica aerogel was nearly two times that of the mesoporous silica gel due to its macroporous structure and its higher silanol content. Silica aerogel was found to be a very promising material for DNA adsorption. Therefore silica aerogel can be considered as a good candidate for the delivery of DNA.
Introduction
Nano and micro particles have many functions such as antibacterial,1 detection in chemical analysis,2 tumor cell targeting,3 polymer composites,4,5 stable semiconductor material6 and DNA delivery systems.7 Recent studies have been focused on development of DNA related nanomaterials and nanotechnologies and the creation of nano-sized structures. Consequently DNA is used for gene therapy, biological sensor preparation, tumor targeting delivery host for targeted drugs, gene and functional nanoscale electronic device, programmable DNA-directed self-assembly film and DNA-conjugated metal nanoparticles.8–15 Besides DNA chips and DNA microarrays are used in molecular biology, pharmaceutical industry and clinical research to identify presence of specific biological targets.16These kinds of technologies require pure DNA usage and understanding of DNA interaction with surfaces. Not only DNA purification but also DNA interactions with different surfaces are directly or indirectly based on the adsorption of DNA. In the case of DNA purification Solid Phase Extraction (SPE) is the most useful and harmless technique for DNA purification. Silica is the most commonly used material for SPE and increasing its adsorption capacity is firstly required.
An inorganic material, typically in the form of amorphous mesoporous silica (SiO2) is one of the preferred matrices for delivery host. On the other hand silica aerogel has been in attention recently because of its tuneable physical properties of pore size, surface area (e.g.).
Nucleic acids are adsorbed on silica and glass surfaces under chaotropic solution conditions because of negatively charged structure of DNA. Its adsorption by silica was extensively investigated.17–19 It is reported that silanol group through the surface enhance the interaction.
Generally both mechanism of DNA adsorption by silica and adsorption capacity are the investigated points. DNA adsorption was controlled by the three effects (i) weak electrostatic repulsion forces, (ii) dehydration, and (iii) hydrogen bond formation. Also the presence of a monovalent cation such as Na+ neutralizes the negative charges on the phosphate backbone of DNA, reducing the electrostatic barrier between DNA and silica.1 Therefore DNA adsorption capacity is increased. Silica particles in microchips16 and miniaturized set up20 were used successfully for solid phase extraction of DNA. There are controversial approaches to the aggregation state of DNA adsorbed on porous solids. DNA fibres having 30–40 nm diameter were observed between the edges and surfaces of particles when Bacillus subtilis DNA was adsorbed on clay. They were not adsorbed as single molecules but as fibres consisting of many molecules.21 However β-type duplex DNA molecules bearing a straight cylindrical structure with a diameter of 2 nm were included in the pore void of M41S type mesoporous silicas having linear cylindrical spaces with diameters in the range from 2 to 5 nm.22 siRNA research was carried out with silica having 3–4 nm diameter pores and 440–450 nm narrow particle size distribution. Because of its high pore volume silica met the requirement of loading small interfering RNA.23
Possibility of inclusion of DNA and siRNA into mesopores of mesoporous silica was investigated by Fujiwara et al.22 and Mayen et al.23 respectively. Mesoporous silicas with 2.80 or 3.82 nm peak pore diameters adsorbed DNA the best in diluted NaCl solution. Formation of the hydrogen bond between P(O)OH groups in DNA and adsorbed water, SiOH groups, or both on silica surfaces was regarded as a main factor in this adsorption. The coincidence of the pore sizes and DNA diameter realizes this unique adsorption promoted by the effect of encompassing DNA with the inner surface of mesoporous silica.
Fortunately sol gel chemistry allows adjusting the pore size of derived materials. The pore dimensions of silica can be obtained in suitable size for loading large molecules. Sol–gel derived amorphous silica (SiO2) is known to be biocompatible. This type of silica was also confirmed as suitable for delivery of drugs, nucleic acids (DNA, siRNA) and proteins.24
Silica aerogel is a special material that has high surface area and porosity.25 Therefore it is also well known nanomaterial with low density, low dielectric constant and isolation properties. It has been used in different scientific research due to its high surface area, open pore structure and biocompatibility since its discovery in 1931.26,27 Space engineering,28 energy collectors,29,30 thermal insulation in solar window systems31 and catalytic supports32,33 are some of the usage areas of silica aerogel. Recently silica aerogel has been used increasingly in biomedical application because of its release properties of enzymes and drugs.34 Drugs, enzymes and proteins are added to silica aerogel during the preparation or the drying procedure.35–38 Not only drug release but also medical application of aerogel has gained attention recently. For example Sabri and co-workers tested long terms of histological evaluation on different intramuscular and subcutaneous aerogel implants on organs.39 Polyurea crosslinked monolithic silica aerogel implants have been found promising for spleen, lung, heart, kidney, and intestine. Without any infection and mild level fibrosis with inflammation silica aerogel has been defined as biocompatible. Its biocompatible property has been also proved for tissue engineering scaffold application of poly-ε-caprolactone composite.40 Aerogel minimize the acidic condition after polymer decomposition. Therefore 3T3 and primary mouse osteoblastic cells were long term stabilized around pH level of 7.2 and 7.4. Biochip for molecular recognition of nucleotide acids is another application of aerogel on biomedical application.41 The aerogel having high surface area was found to be capable of identifying human gene ATP5O.
Aerogel having mesopores was produced under regular atmospheric conditions using the sol–gel process.42 Silica aerogel has the advantage of manipulating its physical properties during preparation.43 These preparation procedures have included three general reversible reactions: hydrolysis-esterification, alcohol condensation-alcoholysis, and water condensation-hydrolysis. Alcogels were formed in the sol–gel process. Therefore the factors that affect the reaction steps change quality and properties of alcogel and indirectly aerogel. Aerogels can be obtained by addition of soluble organic matter during preparation to enlarge the pore diameter42 or by supercritical fluid drying techniques. In supercritical ethanol drying the pores of the alcogel were not collapsed since there were no interfacial tension between the pore walls and gaseous ethanol.
Calf thymus DNA can be used in studying DNA–silica interactions. It is a highly polymerized water soluble polymer with molecular weight higher than 6 × 106 g mol−1.44,45
In the present study characterization of calf thymus DNA in size and charge and its adsorption on mesoporous silica gel and silica aerogel were aimed to elucidate the DNA biosorption. Silica aerogel with a high pore volume and surface area was prepared for adsorption of calf thymus DNA. Supercritical ethanol drying technique was used to obtain the aerogel powder. Both DNA and aerogel were well characterized by advanced analytical techniques before adsorption experiments. Adsorption of DNA from aqueous solutions to the silica aerogel and a mesoporous silica gel was investigated by Nano Drop method, and morphology of freeze dried DNA on silica wafer was investigated by AFM.
Experimental
Materials
Tetraethoxysilane (98%, Aldrich), ethanol (99.8% Riedel, ammonia) (28–30%, Aldrich) were used in preparing silica alcogel. Mesoporous silica gel from Sigma-Aldrich-Silicagel (Grade 7734 pore size 6 nm, particle size 70-230 mesh) was also used for DNA adsorption experiments. Sodium salt of calf thymus DNA supplied by Sigma-Aldrich (D1501) was used in adsorption experiments.Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−3 and led to be stirred for 30 min, and a hydrolysis solution was obtained. In the second step, ammonia, as a base catalyst, was added drop-wise into the solution resulting from the first step, at the molar ratio of TEOS
10−3 and led to be stirred for 30 min, and a hydrolysis solution was obtained. In the second step, ammonia, as a base catalyst, was added drop-wise into the solution resulting from the first step, at the molar ratio of TEOS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4OH equals to 1
NH4OH equals to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−2. The alcogel formed by keeping the mixture for 940 hours at 25 °C was washed several times with ethanol. The aim of this part is to remove excess water present inside the alcogel structure. After preparation of the silica alcogel and the washing procedure, the alcogel having 84.3% ethanol, 1.1% water and 14.6% silica was dried by ethanol supercritical drying.
10−2. The alcogel formed by keeping the mixture for 940 hours at 25 °C was washed several times with ethanol. The aim of this part is to remove excess water present inside the alcogel structure. After preparation of the silica alcogel and the washing procedure, the alcogel having 84.3% ethanol, 1.1% water and 14.6% silica was dried by ethanol supercritical drying.Commercial silicagel, aerogel and DNA morphologies were determined by SEM (FEI Qanta 250 FEG instruments). FTIR spectra of the samples were taken with Shimadzu 8601 FTIR spectrophotometer by KBr disc method to obtain functional group information in the bulk of the samples. The change of surface functional groups with temperature was examined by in situ drift FTIR spectroscopy. Diffuse Reflectance Fourier Transform Infrared (DRIFT) measurements were carried out in an in situ heating reaction cell fitted with CaF2 windows (Harricks, NY) shown in Fig. 3. A praying mantis optical geometry was used to direct the infrared beam. A 0.1 g DNA sample was placed on the sample holder and after its spectrum at room temperature and 100 KPa pressure was taken, the sample chamber was evacuated down to the 0.1 Pa pressure and heated up to 500 °C at a 2 °C min−1 rate at the same pressure. The DRIFT spectra of the sample were obtained at different temperatures during its dynamic heating.
The adsorption of DNA from aqueous solutions at pH 5 on silica aerogel and commercial silica gel were investigated. Before the analysis 100 ng mm−3 calf thymus DNA solution was softly shaken to obtain a homogeneous DNA sample stock solution at pH 5 in acetate buffer solution (ionic strength is 0.001 M). Solutions having 10–90 ng mm−3 DNA were prepared by diluting the stock solution with the buffer solution.
The AFM, Digital Instruments MMSPM Nanoscope IV was used in the tapping mode to obtain the 2 and 3-dimensional surface topology, and phase information of the surfaces in air. For imaging in air, Veeco RTESP tips were used. The tips had anisotropic geometry, with tip height 15–20 μm and tip radius less 10 nm. The spring constant of the cantilever was 40 N m−1. Nominal length and width of the cantilever were 125 and 35 μm respectively. The typical tapping frequency was 263–291 kHz, the scanning rate was 1.0 Hz and scan angle was zero degrees.
Results and discussion
DNA characterization
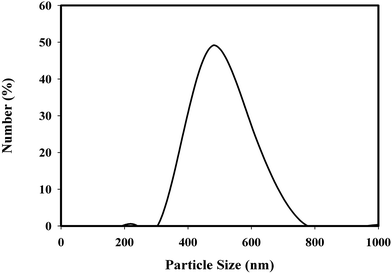
Considering the information of 0.34 nm helix rise per turn, 10.5 base pair per helical turn for the double helix of B-DNA52 and the average molar mass of a base pair as 650 g mol−1, the length of calf thymus DNA molecules should be 300 and 400 nm for 6 × 106 and 8 × 106 g mol−1 molar−1 mass of DNA reported in ref. 44 and 45. If the self-assembled fibres in dry form were dispersed into their individual molecules, particles having 2 nm diameter and 4.18 and 3.18 μm length should be present in aqueous solutions. Since there is a lower limit of sizes that can be determined by the Zetasizer, the particle size distribution shown in Fig. 7 should give information about the length of the fibres not about their diameter. DNA in solution had the particle size distribution shown in Fig. 7. The particle size ranged from 300 to 800 nm and it was 490 nm on the average as seen in Fig. 7. The breadth of the distribution curve at half height was 230 nm.
Silica characterization
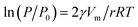 | (1) |
| Properties | Silica gel | Aerogel |
|---|---|---|
| Single point surface area (m2 g−1) | 556 | 1055 |
| BET surface area (m2 g−1) | 571 | 1107 |
| Langmuir surface area (m2 g−1) | 787 | 1549 |
| Average pore diameter (nm) (4V/A by BET) | 5.5 | 4.2 |
| Total pore volume (cm3 g−1) (single point from N2 adsorption) | 0.79 | 1.19 |
| (From tapped bulk density measurements) | 0.74 | 7.00 |
| Max micropore volume (cm3 g−1) | 0.20 | 0.36 |
| Mesopore volume (cm3 g−1) | 0.59 | 0.73 |
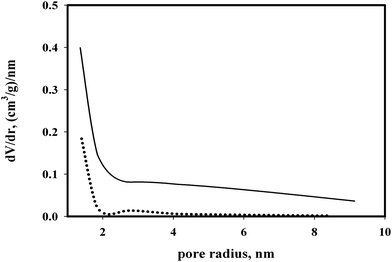
Only micropore and mesopore volumes can be determined by N2 adsorption. Thus the total pore volume should be determined using other methods. The tapped bulk densities of the mesoporous silica gel and silica aerogel were determined as 0.56 g cm−3 and 0.09 g cm−3 respectively. Considering random packing particles void volume fraction as 0.33 and density of silica as 2.2 g cm−3 the total pore volumes were calculated as 0.73 and 7 cm3 g−1 respectively for mesoporous silica gel and silica aerogel respectively. While the total pore volumes determined by nitrogen adsorption and from bulk density measurements were close to each other for mesoporous silica gel, they were different from each other for silica aerogel. The silica aerogel had pore volume of 7 and 1.19 cm3 g−1 from bulk density measurement and from N2 adsorption respectively. Thus there were macropores in silica aerogel which would allow the entrance of large DNA particles.
Mesoporous silica had lower concentration of silanol (Si–OH) groups since the ratio of the absorbance values at 950 cm−1/1100 cm−1 are 0.13 and 0.15 respectively for mesoporous silica gel and aerogel respectively. Silica aerogel adsorbed more DNA than mesoporous silica gel; since DNA was bonded to silica surface through silanol groups,21 silica aerogel was macroporous and had higher surface area.
 | ||
Fig. 15 Adsorption isotherm of DNA on silica aerogel and silica gel at pH 5 aerogel experimental (●), silicagel experimental (▲), aerogel model (Freunlich) (…), silica gel model (Langmuir) (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ). ). | ||
The adsorption of self-assembled DNA on silica aerogel and silica gel should be a complicated process. Langmuir and Freunlich adsorption models developed for adsorption of small molecules on solid surfaces were applied to DNA adsorption as an over simplification. The Langmuir model shown in eqn (2) assumes monolayer adsorption onto homogeneous surfaces, whereas the semiempirical Freundlich model in eqn (3) works well for heterogeneous surfaces at low concentrations that do not show a finite uptake capacity.
 | (2) |
 | (3) |
Langmuir and Freundlich model graphs were drawn for silica aerogel and mesoporous silica gel. Both isotherm models fit to adsorption data with R2 values higher than 0.8. R2 values were higher for Langmuir and Freundlich models for mesoporous silica gel and silica aerogel respectively. However R2 value was higher, (0.96) for Freundlich model for silica aerogel, and it was higher (0.90) for Langmuir model for silica gel as reported in Table 2.
| Langmuir Model | Freundlich model | |||||
|---|---|---|---|---|---|---|
| Qm (μg g−1) | b (cm3 μg−1) | R2 | n | Kf (μg1−n g−1 cm−3n) | R2 | |
| Silica gel | 1428 | 0.031 | 0.90 | 1.67 | 94 | 0.83 |
| Aerogel | 2500 | 0.037 | 0.84 | 2.30 | 211 | 0.96 |
The Langmuir adsorption capacities of DNA with 7000 base pairs to silica with 3.4, 5.4, 10.0 nm pore sizes and treated with Mg2+ ions were 2.49, 5.71 and 15.7 μg mg−1 respectively. Silica with 5.4 nm pore size and treated with Na+ ions had a smaller adsorption capacity, only 0.22 μg mg−3. On the other hand salmon sperm DNA was adsorbed in the 0.5–2.7 μg mg−1 range on mesoporous silica with different pore sizes at pH value of 7.43 in 0.005 mol dm−3 NaCl solution.6 The aerogel synthesized in the present study had 2.5 μg mg−1 adsorption capacity at pH 7 for highly polymerized DNA.
Discussion
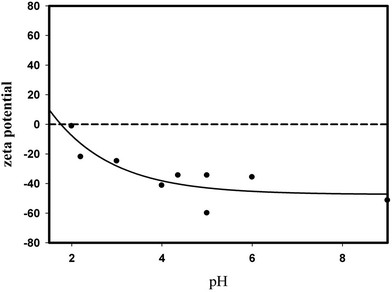
Adsorption is a process that should be taken in consideration of both the adsorbate and the adsorbent. Therefore characterization of the adsorbate calf thymus and mesoporous silica and synthesised aerogel adsorbents are important. DNA dispersion in water is important in determining the size of the pores in the adsorbent which they can enter. Thus the size of the DNA agglomerates both by Zetasizer and AFM. Freeze drying on silica wafer allowed us to determine the both morphology of the DNA on flat silica surface and its original dimensions in water. Zeta potential of DNA and silica at the adsorption pH were both negative, thus the adsorption was due to van der waals attraction and hydrogen bonding of DNA with Si–OH groups. Thus synthesizing aerogel with macropores and higher silanol content allowed adsorption of large agglomerates of DNA in silica.DNA dissolved in water was not present as single molecules in the present study on contrary to the observations of Fujiwara et al.22 DNA dissolved in water had 490 nm average size as determined by Zetasizer. AFM studies indicated the presence of self-assembled super helices with 2.85 ± 0.74 μm length and with 0.10 ± 0.1 μm average diameter and 0.15 μm pitch. Since highly polymerized calf thymus DNA in the aqueous phase was present as self-assembled large particles they could only be adsorbed in macropores and on the external surface of the silica particles rather than its micro and mesopores. Bacillus subtilis DNA was also adsorbed in fibre form on another adsorbent, clay21 similar to calf thymus DNA on silica wafer.
Silica aerogel had smaller pore diameter (4.2 nm) than that of silica gel (5.5 nm) in micro and mesopore region. On the other hand the surface area of silica aerogel (1107 m2 g−1) was higher than that of mesoporous silica gel (571 m2 g−1). If the size (490 nm by Zetasizer, 2.85 μm in length, 100 nm in diameter by AFM) of the DNA particles dispersed in water is considered, the entrance of DNA in the micro and meso pores was not possible. Thus the higher adsorption capacity of aerogel could be explained by the presence of macropores and the higher concentration of Si–OH groups in aerogel.
After successful initial stage adsorption, the next stage is investigation of desorption. DNA was desorbed from mesoporous silica with magnetic particles by using an elution buffer with 150 mM NaCl and by increasing temperature to 37 °C.55
DNA made hydrogen bonds with silanol groups on adsorption and the hydrogen bonds were broken during desorption55 DNA was adsorbed both in the external surface and in the mesopores. DNA in mesopores required a higher temperature than DNA on external surface to desorption to eluting buffer indicating stronger hydrogen bond formation in mesopores than the external surface.55
Conclusions
A silica aerogel was obtained by supercritical ethanol drying technique in the present study. It had a higher surface area and total pore volume than a mesoporous silica gel obtained by conventional drying. The adsorption of highly polymerized calf thymus self-assembled DNA in the aqueous phase should occur on the macropores and the external surface of the silica particles rather than its pores. Silica aerogel had nearly two times the adsorption capacity than the mesoporous silica gel. DNA adsorption onto silica surface is related with silanol group and silanol group is directly proportional with surface area. In situ drift FTIR spectroscopy indicated the presence of isolated OH groups in silica aerogel. The higher content of silanol groups and presence of macropores had positive effects on DNA adsorption capacity in silica aerogel. While DNA adsorption in silica aerogel best fitted to Freundlich isotherm with Kf and n constants of 211 and 2.3 respectively, it fitted to Langmuir model with Qm 1428 ng mg−1 and b 0.031 cm3 μg−1 for mesoporous silica gel. Adsorption sites in silica aerogel had a distribution of energies (Freundlich isotherm), while in mesoporous silica gel equal energy adsorption sites (Langmuir) were present.DNA adsorption is the initial stage of gene therapy for drug delivery and DNA purification system. High pore volume and high adsorption capacity are the simple requirements of producing ‘smart’ drug delivery system and kit. Consequently silica aerogel was found to be a very promising material for DNA adsorption. Silica aerogel therefore presents an excellent material for a short-term DNA delivery system provided it can be made to release DNA in a safe and efficacious manner into living vasculature and/or tissues.
Notes and references
- C. Dong, Z. Yan, J. Kokx, D. B. Chrisey and C. Z. Dinu, Appl. Surf. Sci., 2012, 258, 9218–9222 CrossRef CAS PubMed.
- Q. Xu, P. Pu, J. Zhao, C. Dong, C. Gao, Y. Chen, J. Chen, Y. Liua and H. Zhoua, J. Mater. Chem. A, 2015, 3, 542–546 CAS.
- Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883–2886 CrossRef CAS PubMed.
- E. Sahin, F. Y. Mahlicli, S. Yetgin and D. Balkose, J. Appl. Polym. Sci., 2012, 125, 1448–1455 CrossRef CAS PubMed.
- S. Yetgin, S. Ulutan and D. Balkose, J. Vinyl Addit. Technol., 2015, 21(1), 42–50 CrossRef CAS PubMed.
- T. O. Egbuchunam, S. Yetgin, F. O. Omurlu and D. Balkose, Int. J. Appl. Eng. Res., 2014, 9(21), 11631–11646 Search PubMed.
- D. D. Dunlap, A. Maggi, M. R. Soria and L. Monaco, Nucleic Acids Res., 1997, 25(15), 3095–3101 CrossRef CAS PubMed.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Stofhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- E. Winfree, F. Liu, L. A. Wenzler and N. C. Seeman, Nature, 1998, 394, 539–544 CrossRef CAS PubMed.
- E. Braun, Y. Eichen, U. Sivan and G. Ben-Joseph, Nature, 1998, 391, 775–778 CrossRef CAS PubMed.
- H. W. Fink and C. Schonenberger, Nature, 1999, 398, 407–410 CrossRef CAS PubMed.
- Z. Yu, R. M. Schmaltz, T. C. Bozeman, R. Paul, M. J. Rishel, K. S. Tsosie and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 2883–2886 CrossRef CAS PubMed.
- Z. GEstephan, Z. Qian, D. Lee, J. C. Crocker and S. J. Park, Nano Lett., 2013, 13, 4449–4455 CrossRef PubMed.
- X. J. Chen, B. L. Sanchez-Gaytan, Z. Qian and S. J. Park, Nanomed. Nanobiotechnol., 2012, 4, 273–290 CrossRef CAS PubMed.
- P. Stoliar, E. Bystrenova, S. D. Quiroga, P. Annibale, M. Facchini, M. Spijkman, S. Setayesh, D. de Leeuw and F. Biscarini, Biosens. Bioelectron., 2009, 24, 2935–2938 CrossRef CAS PubMed.
- M. C. Breadmore, K. A. Wolfe, I. G. Arcibal, W. K. D. Leung Dickson, B. C. Giordano, M. E. Power, J. P. Ferrance, S. H. Feldman, P. M. Norris and J. P. Landers, Anal. Chem., 2003, 75, 1880–1886 CrossRef CAS.
- K. A. Melzak, C. S. Sherwood, R. B. F. Turner and C. A. Haynes, J. Colloid Interface Sci., 1996, 181, 635 CrossRef CAS.
- Y. Mao, L. Y. Daniel, N. Whittaker and U. Saffiottil, Environ. Health Perspect., 1994, 102, 165 CrossRef CAS.
- S. M. Solberg and C. C. Landry, J. Phys. Chem. B, 2006, 110, 15261–15268 CrossRef CAS PubMed.
- H. Tian, A. F. R. Huhmer and J. P. Landers, Anal. Biochem., 2000, 283, 175–191 CrossRef CAS PubMed.
- M. Khanna, M. Yoder, L. Calamai and G. Stotzky, Sci. Soils, 1998, 3, 1–10 CrossRef PubMed.
- M. Fujiwara, F. Yamamoto, K. Okamoto, K. Shiokawa and R. Nomura, Anal. Chem., 2005, 77, 8138–8145 CrossRef CAS PubMed.
- V. Mayen, T. Wutikhun, O. Ketchart and P. Kopermsub, Journal of the Microscopy Society of Thailand, 2012, 5, 10–13 Search PubMed.
- D. Avnir, T. Coradin, O. Lev and J. Livage, J. Mater. Chem., 2006, 16, 1013–1030 RSC.
- A. S. Dorcheh and M. H. Abbasi, J. Mater. Process. Technol., 2008, 199, 10–26 CrossRef PubMed.
- S. S. Kistler, Nature, 1931, 127–741 Search PubMed.
- L. W. Hrubesh, J. Non-Cryst. Solids, 1998, 225, 335–342 CrossRef CAS.
- M. A. B. Meador, E. F. Fabrizio, F. Ilhan, G. A. Dass, P. Zhang, J. C. Vassilaras and N. L. Johnston, Chem. Mater., 2005, 17, 1085–1098 CrossRef CAS.
- M. Reim, A. Beck, W. Korner, R. Petricevic, M. Glora, M. Weth, T. Schliermann, J. Fricke, C. Schmidt and F. J. Potter, Sol. Energy, 2002, 2, 21 CrossRef.
- M. Reim, G. Reichenauer, W. Körner, J. Manara, M. Arduini-Schuster and S. Korder, J. Non-Cryst. Solids, 2004, 350, 358–363 CrossRef CAS PubMed.
- S. A. Omer, S. B. Riffat and G. Qiu, Building Services Engineering Research and Technology, 2007, 28, 275 CrossRef PubMed.
- A. C. Pierre and G. M. Pajonk, Chem. Rev., 2002, 102, 4243 CrossRef CAS PubMed.
- J. Estella, J. C. Echeverrıa, M. L. Julian and J. Garrido, J. Porous Mater., 2008, 15, 705 CrossRef CAS.
- M. Alnaief and I. Smirnova, J. Non-Cryst. Solids, 2010, 356, 1644–1649 CrossRef CAS PubMed.
- P. Buisson, C. Hernandez, M. Pierre and A. C. Pierre, J. Non-Cryst. Solids, 2001, 285, 295–302 CrossRef CAS.
- I. Smirnova, J. Mamic and W. Arlt, Langmuir, 2003, 19, 8521–8525 CrossRef CAS.
- I. Smirnova, S. Suttiruengwong and W. Arlt, J. Non-Cryst. Solids, 2004, 350, 54–60 CrossRef CAS PubMed.
- Y. K. Li, M. J. Chou, T. Y. Wu, T. R. Jinn and Y. W. Chen-Yanga, Acta Biomater., 2008, 4, 725–732 CrossRef CAS PubMed.
- F. Sabri, J. D. Boughter Jr, D. Gerth, O. Skalli, T. N. Phung, G. R. M. Tamula and N. Leventis, PLoS One, 2012, 7(12), e50686, DOI:10.1371/journal.pone.0050686.
- J. Ge, M. Li, Q. Zhang, C. Z. Yang, P. H. Wooley, X. Chen and S. Yang, Silica Aerogel Improves the Biocompatibility in a Poly-ε-Caprolactone Composite Used as a Tissue Engineering Scaffold, Int. J. Polym. Sci., 2013 DOI:10.1155/2013/402859.
- Y. K. Li, D. K. Yang, Y. C. Chena, H. J. Suc, J. C. Wub and Y. W. Chen-Yanga, Acta Biomater., 2010, 6, 1462–1470 CrossRef CAS PubMed.
- J. Martin, B. Hosticka and P. Norris, J. Non-Cryst. Solids, 2001, 285, 222–229 CrossRef CAS.
- M. Mukhopadhyay and B. S. Rao, J. Chem. Technol. Biotechnol., 2008, 83, 1101–1109 CrossRef CAS PubMed.
- M. Tanigawa, M. Suzuto, K. Fukudome and K. Yamaoka, Macromolecules, 1996, 29, 7418–7425 CrossRef CAS.
- N. Sundaresan, H. C. Suresh, T. Thomas, T. J. Thomas and C. K. S. Pillai, Biomacromolecules, 2008, 9, 1860–1869 CrossRef CAS PubMed.
- Y. Ru, L. Guoqiang and L. Min, Microporous Mesoporous Mater., 2010, 129, 1 CrossRef CAS PubMed.
- A. Wu, Z. Li and E. Wang, Anal. Sci., 2004, 20, 1083–1086 CrossRef CAS.
- H. Zhao, J. Chem. Technol. Biotechnol., 2015, 90, 19–25 CrossRef CAS PubMed.
- P. Cai, Q. Huang, D. Jiang, X. Rong and W. Liang, Colloids Surf., B, 2006, 49, 49–54 CrossRef CAS PubMed.
- G. Stotzky, J. Environ. Qual., 2000, 29, 691–705 CrossRef CAS.
- F. Ke, Y. K. Luu, M. Hadjiargyrou and D. Liang, Characterizing DNA Condensation and Conformational Changes in Organic Solvents, PLoS One, 5(10), e13308, DOI:10.1371/journal.pone.0013308.
- D. L. Nelson and C. M. M. Lenhinger, Principles of Biochemistry, Wroth, NewYork, 2000 Search PubMed.
- A. Fidalgo and L. M. Ilharco, Microporous Mesoporous Mater., 2005, 84, 229–235 CrossRef CAS PubMed.
- P. Munnik, M. Wolters, A. Gabrielsson, S. D. Pollington, G. Headdock, J. H. Bitter de, P. E. Jongh and K. P. de Jong, J. Phys. Chem. C, 2011, 115, 14698–14706 CAS.
- X. Li, J. Zhang and H. Gu, Langmuir, 2011, 27, 6099–6106 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |