The energy band tailored by Al incorporation in solution-processed IZO TFTs
Abstract
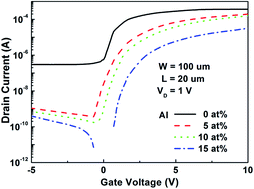
The energy band tailoring of indium zinc oxide (IZO) through Al incorporation was studied and measured directly by ultraviolet photoelectron spectroscopy (UPS). Al doped IZO (AIZO) thin films have lower work function (3.90 eV) and wider bandgap (3.75 eV) compared with IZO (4.15 and 3.5 eV, respectively). These changes induced by Al incorporation would result in higher activation energy and higher flat voltage for AIZO TFTs, which may imply the origin of the AIZO TFTs electrical properties such as threshold voltage shift and off-state current decrease.

 Please wait while we load your content...
Please wait while we load your content...