Synthesis and characterization of gold complexes with pyridine-based SNS ligands and as homogeneous catalysts for reduction of 4-nitrophenol†
Wei-Guo Jia*,
Yuan-Chen Dai,
Hai-Ning Zhang,
Xiaojing Lu and
En-Hong Sheng
College of Chemistry and Materials Science, Center for Nano Science and Technology, The Key Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Laboratory of Molecular-Based Materials, Anhui Normal University, Wuhu, 241000, China. E-mail: wgjiasy@mail.ahnu.edu.cn
First published on 20th March 2015
Abstract
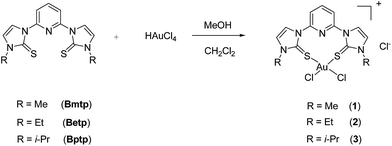
Three gold complexes with pyridine-based SNS type ligands, 2,6-bis(1-methylimidazole-2-thione)pyridine (Bmtp), 2,6-bis(1-ethylimidazole-2-thione)pyridine (Betp) and 2,6-bis(1-isopropylimidazole-2-thione)pyridine (Bptp) have been synthesized and characterized. Reactions of HAuCl4·H2O with three pyridine-based SNS ligands result in the formation of the complexes [(L)AuCl2]Cl (L = Bmtp (1); L = Betp (2) and L = Bptp (3)). All compounds are investigated by elemental analysis, NMR, MS and UV-Vis as well as IR spectral analysis. The molecular structure of 3 has been confirmed by X-ray crystallography. Moreover, all gold complexes are efficient homogeneous catalysts and catalyze the reduction of 4-nitrophenol (4-NP) to 4-nitroaniline (4-AP) in the presence of NaBH4 reducing agent in water. And complex 3 exhibits an exceptionally high turn over frequency (TOF) of 1.20 min−1 for the reduction of 4-NP.
Introduction
Gold-based compounds including complexes and nanomaterials (particles, boxes, cages and tubes) have exhibited extraordinarily rich catalytic activity for a variety of reactions involving oxidation and reduction due to their unique physicochemical properties over the past few decades.1,2 The interest of exploring gold-based catalysts has been substantially booming. For heterogeneous gold-based catalytic systems, a high dispersion of gold nanoparticles is necessary to exhibit high catalytic activity. Subsequently, many solid supporting gold catalysts including polymer,3 hydrogel,4 silica particles,5 metal oxides6 and magnetic materials7 have been developed.2 In contrast to the heterogeneous catalytic system, homogeneous gold catalysts have been found to be very effective for construction of C–C and C–N bonds through C–H activation.8 The mechanisms of the heterogeneous reactions are not yet well understood, whereas the mechanisms have been well explored in the case of the homogeneous reactions. Therefore, the design and synthesis of homogeneous gold catalysts with stable and high catalytic efficiency has received increasing attention recently.In previous studies, we synthesized Ir and Rh complexes based on organochalcogen ligands (SS, SeSe and SNS) and studied their catalytic activities in norbornene polymerization.9,10 Organochalcogen chemistry revolving transition metal complexes is one of research focuses of our groups, and we seek to discover new transition metal complexes and study their potential catalytic applications. Herein, the gold complexes with three pyridine-based SNS ligands [(L)AuCl2]Cl (L = Bmtp (1); L = Betp (2) and L = Bptp (3)) have been synthesized and characterized. Moreover, the molecular structure of 3 has been determined by X-ray crystallography. The various organochalcogen ligands were selected in order to investigate the influence of alkyl substituents on imidazole ring with different electronic properties on catalytic properties of the resulting complexes. The results show that all gold complexes are efficiently catalyze 4-NP reduction to 4-AP in the presence of NaBH4 reducing agent under homogeneous conditions. Furthermore, the steric and electronic effects of the alkyl group in the organochalcogen ligands have a strong influence on the 4-NP reduction (i-Pr > Et > Me).
Results and discussion
Synthesis of ligands and gold complexes
Three pyridine-based SNS ligands, 2,6-bis(1-methylimidazole-2-thione)pyridine (Bmtp), 2,6-bis(1-ethylimidazole-2-thione)pyridine (Betp) and 2,6-bis(1-isopropylimidazole-2-thione)pyridine (Bptp) were prepared in one-pot via the reactions of pyridine bridged imidazolium dibromide derivatives with sulfur powder in the presence of K2CO3 according to the previous literature method (Scheme 1).10,11 All these compounds were stable towards air and moisture in the solid state, and exhibit good solubility in common organic solvents. All compounds were characterized by NMR, IR spectroscopy as well as elemental analysis. The 1H NMR spectrum of Betp show signals at δ 1.42, 4.18, 6.82, 7.49, 8.02 and 8.88 ppm, which can be assigned to the alkyl, imidazole, and pyridyl groups, respectively. And the 13C NMR spectra show singlet at about δ 161.8 ppm for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group in Betp, which is also consistent with Bmtp and Bptp.
S group in Betp, which is also consistent with Bmtp and Bptp.
When HAuCl4 was treated with three pyridine-based SNS ligands: Bmtp, Betp and Bptp in MeOH at room temperature, the orange crystals of gold complexes (1–3) were isolated in moderate yields as air and moisture-stable, respectively (Scheme 2). Complexes 1–3 were fully characterized by IR, NMR spectroscopy, elemental analysis as well as MS. All these gold complexes have similar characteristic peaks on NMR spectra, so it is feasible to take 1 for an example. The 1H NMR spectrum of 1 show signals at δ 4.13, 7.75, 7.85, 7.94 and 8.47 ppm, which can be assigned to the methyl, imidazole, and pyridyl groups, respectively. Likewise, upfield shifts of up to 15.4 ppm for the thione carbons in their 13C NMR were measured upon complexation to gold metal, which also prove the formation of the gold complex.
Crystals of 3 suitable for X-ray crystallographic diffraction were obtained by slow diffusion of diethyl ether into a concentrated solution of the complex in methanol solution.
Crystallographic data and details of data collection and refinement for complex 3 are summarized in Table 1, the important bond lengths and angles are given in Table 2. The molecular structure of 3 is shown in Fig. 1.
| 3 | |
|---|---|
| a R1 = ∑||Fo| − |Fc||/∑|Fo|; wR2 = [∑w(|Fo2| − |Fc2|)2/∑w|Fo2|2]1/2. | |
| Empirical formula | C18H21AuCl3N5O2S2 |
| Formula weight | 706.830 |
| Crystal syst., space group | Triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 7.981 (4) |
| b (Å) | 11.981 (6) |
| c (Å) | 15.652 (8) |
| α (°) | 106.507 (6) |
| β (°) | 104.098 (6) |
| γ (°) | 97.096 (6) |
| Volume (Å3), Z | 1361.5 (12), 2 |
| Dc (mg m−3) | 1.724 |
| μ (Mo-Kα) (mm−1) | 5.873 |
| F(000) | 684 |
| θ Range (°) | 1.81–27.62 |
| Limiting indices | −10, 10; −15, 14; −20, 20 |
| Reflections/unique [R(int)] | 6033/2740 [0.0988] |
| Completeness to θ (°) | 27.62 (95.2%) |
| Data/restraints/parameters | 6033/0/243 |
| Goodness-of-fit on F2 | 0.956 |
| Final R indices [I > 2σ(I)]a | R1 = 0.0797, wR2 = 0.1770 |
| R Indices (all data) | R1 = 0.1977, wR2 = 0.2250 |
| Au1–S1 | 2.340(5) | Au1–S2 | 2.332(4) |
| Au1–Cl1 | 2.320(4) | Au1–Cl2 | 2.315(5) |
| S1–Au1–S2 | 88.86(15) | S1–Au1–Cl1 | 90.10(17) |
| S1–Au1–Cl2 | 178.51(17) | S2–Au1–Cl1 | 178.08(19) |
| S2–Au1–Cl2 | 89.80(16) | Cl1–Au1–Cl2 | 91.22(18) |
The molecular structure of 3 is solved in the triclinic crystal system and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. As can be seen, the coordination geometry of the central gold center can be best described as a nearly square-planar geometry with two Cl atoms and two N atoms (S1–Au1–S2 88.86(15)°, S1–Au1–Cl1 90.10(17)°, S1–Au1–Cl2 178.51(17)°, S2–Au1–Cl1 178.08(19)°, S2–Au1–Cl2 89.80(16)° and Cl1–Au1–Cl2 91.22(18)°). Both sulfur atoms and two chlorine atoms of Bptp ligand in a cis disposition. Bond distances around the gold ion are as expected (Au–Clav = 2.318 Å and Au–Sav = 2.336 Å) and are comparable to the distances observed in the [AuCl2(S2CNBn2)] (Au–Cl = 2.3116(15) Å, Au–S = 2.3005(14) Å).12
space group. As can be seen, the coordination geometry of the central gold center can be best described as a nearly square-planar geometry with two Cl atoms and two N atoms (S1–Au1–S2 88.86(15)°, S1–Au1–Cl1 90.10(17)°, S1–Au1–Cl2 178.51(17)°, S2–Au1–Cl1 178.08(19)°, S2–Au1–Cl2 89.80(16)° and Cl1–Au1–Cl2 91.22(18)°). Both sulfur atoms and two chlorine atoms of Bptp ligand in a cis disposition. Bond distances around the gold ion are as expected (Au–Clav = 2.318 Å and Au–Sav = 2.336 Å) and are comparable to the distances observed in the [AuCl2(S2CNBn2)] (Au–Cl = 2.3116(15) Å, Au–S = 2.3005(14) Å).12
As shown in the Fig. 2, in complex 3, there are two kinds of weak intermolecular C–H⋯Cl hydrogen bonds between chlorine anion and carbon of ligand. One is C–H⋯Cl hydrogen bond (C(4)–C1(3) = 3.49(2) Å, C(4)–H(4)⋯Cl(3) = 169°) and the other is C–H⋯Cl hydrogen bond (C(13)–C1(3) = 3.484(14) Å, C(13)–H(13)⋯Cl(3) = 174°). These weak intramolecular hydrogen bonds play a crucial role in the construction of 1D polymeric chain structure in the solid states. And the similar weak interactions have been found among the transition metal coordination complexes.13
Catalytic degradation of 4-NP
Catalytic reduction of 4-NP is possibly the most often used reaction to test the catalytic activity of gold-based catalysts in aqueous solution.14 4-NP is one of the most common organic pollutants found in dye industrial and agricultural wastewaters. The reduction of 4-NP by gold-based catalysts in the presence of NaBH4 reducing agent leads to the formation of 4-AP, which is an useful compound for a wide range of applications include pharmaceuticals, corrosion treatments, and so on.15 The catalytic performance of the gold complexes were evaluated in the reduction of 4-NP in the presence of NaBH4 under homogeneous conditions in water. The catalytic reactions were monitored by UV-Vis spectroscopy, by recording the UV spectra of aliquots withdrawn from the reaction medium at 1 min intervals. In Fig. 3 are presented the time-dependent UV-Vis spectra of the 4-NP reduction catalyzed by gold complexes catalysts. As shown in Fig. 3, upon the increase of the reaction time, the intensity of the absorption band at λ = 400 nm from 4-nitrophenolate ion becomes weaker, and at the same time, an absorption band around λ = 300 nm appears due to the formation of 4-AP, which increases in intensity during the course of the reaction.Fig. 3(A–C) provides the time-dependent UV-Vis adsorption spectra of the reaction mixture catalyzed by gold complexes. All gold catalysts are highly active in the 4-NP reduction, with the reaction catalyzed by complex 1 reaching the maximum substrate conversion of 93.2% within 13 minutes, whereas for the reaction catalyzed by complex 2 and 3 the maximum conversion of 97.5% and 96.5% were achieved after 11 and 8 minutes of reaction time, respectively. Since the concentration of NaBH4 is much higher than that of 4-NP (100-fold times, see ESI†), the rate of reduction of 4-AP is independent of the NaBH4 concentration which allows this reaction to be evaluated by pseudo-first-order kinetics. The kinetic profiles of the 4-NP reduction using all gold catalysts are presented in Fig. 3(D), through the representation of ln(Ct/C0) as a function of the reaction time, where Ct and C0 are the concentration of 4-NP at the beginning and at a certain reaction time respectively. Fig. 3(D) shows the ln(Ct/C0) versus time for all gold catalysts systems and good linear relationships are observed, indicating that the reduction of 4-NP to 4-AP follows the pseudo-first-order kinetics. The kinetic reaction rate constants (k) were determined from the slope of the straight line which is 2.6 × 10−3 S−1 for complex 1, 2.7 × 10−3 S−1 for complex 2 and 5.1 × 10−3 S−1 for complex 3, respectively. This result means that complex 3 possess the faster reduction rate and thus higher catalytic activity for the reduction of 4-NP, which should be attributed to the size and electronic effect of alkyl substituent on imidazole ring in gold complexes (i-Pr > Et > Me).
In order to evaluate the catalytic results, we use the turnover frequency (TOF) to determine the efficiency of our gold complexes catalysts for 4-NP reduction and compare with previously reported gold based catalysts. The TOF of complex 3 catalyst is 1.20 min−1, calculated by the moles of 4-NP reduced per mole of gold complex per consumed time under the present reaction conditions. As shown in Table 3, in comparison with other gold-based catalysts, our gold complexes catalysts showed a higher catalytic efficiency than most of other gold-based catalysts for the reduction of 4-NP. The above results showed that gold complexes with pyridine-based SNS ligands possessed superior high catalytic activity, which should be attributed to the homogeneous catalytic system.
| Catalysts | TOF (min−1) | Catalysts (mol%) | References |
|---|---|---|---|
| Complex 1 | 0.71 | 10 | This work |
| Complex 2 | 0.88 | 10 | This work |
| Complex 3 | 1.20 | 10 | This work |
| Au(0)@TpPa-1 | 0.17 | 45 | 16 |
| HAuCl4·3H2O | 0.11 | 46 | 16 |
| Au–PMMA | 1.53 | 7 | 17 |
| Fe3O4@SiO2–Au@mSiO2 | 0.73 | 9 | 18 |
| Fe3O4@P(EGDMA-co-MAA)/Au | 1.6 | 2.5 | 19 |
| Au NPs/SNTs | 0.77 | 28 | 20 |
| PDEAEMA–AuNP | 0.47 | 57 | 21 |
| Au@SiO2 | 0.45 | 7 | 22 |
The UV-Vis absorbance of gold complexes
The absorption spectra of all gold complexes and ligands measured at room temperature in MeOH solvents were given in Fig. 4. All ligands and gold complexes show intense absorptions at 208 nm which can be assigned to π–π* of pyridine-based SNS ligand. The peaks at 225 and 321 nm were found to become weak in gold complexes, which may be attributed to the charge transfer between pyridine-based SNS ligand and Au(III) metal centre.Conclusion
In conclusion, we have reported three gold complexes with pyridine-based ligands. A combination of spectroscopic studies and X-ray crystallographic confirmed the molecular structures of gold complexes. And all gold complexes are highly catalyze 4-NP reduction to 4-AP in the presence of NaBH4 reducing agent under homogeneous conditions in water. And the complex 3 exhibits an exceptionally high TOF of 1.20 min−1 for reduction of 4-NP.Experimental section
General
All manipulations were carried out under nitrogen using standard schlenk and vacuum-line techniques. All solvents were purified and degassed by standard procedures. The starting materials, Bmtp and Bptp were synthesized according to the procedures described in the literature.10,11 Other chemicals were analytical grade and used as received. 1H and 13C NMR were recorded on a 300 MHz or 500 MHz NMR spectrometer at room temperature. Chemical shifts (δ) are given in ppm relative to internal TMS and are internally referenced to residual 1H and 13C solvent resonances. IR spectra were recorded on a Nicolet AVATAR-360IR spectrometer. Element analyses were performed on an Elementar III vario EI Analyzer. Mass spectrometry was performed on Bruker BIFLEX III MALDI-TOF-MS instrument.Synthesis of Betp
In a 100 mL round-bottomed flask fitted with reflux condenser were placed 2,6-bis(1-ethylimidazolium)pyridine dibromide (3.31 g, 10 mmol), S (0.64 g, 20 mmol), K2CO3 (2.8 g, 20 mmol) and 50 mL methanol as solvent. The mixture was allowed to reflux for 8 h after which the methanol was removed with a rotary evaporator. The remaining solid was shaken with 2 × 30 mL CH2Cl2 which was then filtered and rotary evaporated. The product was recrystallized from CH2Cl2/MeOH to give colorless solid. Yield: (1.92 g, 58%) Anal. Calcd for C15H17N5S2 (331.09): C, 54.37; H, 5.17; N, 21.15. Found: C, 54.30; H, 5.25; N, 21.30. 1H NMR (500 MHz CDCl3): 8.88 (d, J = 8 Hz, pyridine, 2H), 8.02 (t, pyridine, 1H), 7.49 (d, J = 3 Hz, imidazole, 2H), 6.82 (d, J = 3 Hz, imidazole, 2H), 4.18 (m, 2CH24H), 1.42 (t, 2CH3, 6H) ppm. 13C NMR (500 MHz, CDCl3): δ 161.8, 148.4, 139.7, 117.0, 116.3, 116.8, δ 42.7, 13.9 ppm. IR (KBr cm−1): 1143 (m) (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S).
S).
Synthesis of complex 1
In a 100 mL round-bottomed flask were placed Bmtp (152 mg, 0.5 mmol), HAuCl4 (206 mg, 0.5 mmol), 30 mL methanol as solvent. The mixture was stirred at room temperature overnight and then the solvent was removed with a rotary evaporator; the resulting solid was washed with methanol, and then dried in vacuo. The product was recrystallized from MeOH/hexane to give orange powder. Yield: (137 mg, 45%). Anal. Calcd for C13H13Cl3N5S2Au (606.73): C, 25.79; H, 2.17; N, 11.57. Found: C, 25.90; H, 2.28; N, 11.66. 1H NMR (300 MHz, CD3CN) δ 8.47 (t, pyridine, 1H), 7.94 (d, J = 2.1 Hz, imidazole, 2H), 7.85–7.87 (d, pyridine, 2H), 7.75 (d, J = 2.1 Hz, imidazole, 2H), 4.13 (s, 6H). 13C NMR (500 MHz, CD3CN) δ 147.54, 144.90, 125.90, 123.95, 121.80, 30.29. MS (MALDI-TOF): calcd for C13H13N5S2Au3+ 500.0278 (M3+), found 500.1567 (M3+). IR (KBr cm−1): 3451 (m), 3136 (w), 3114 (w), 3077 (w), 1595 (m), 1551 (w), 1477 (s), 1400 (w), 1317 (w), 1239 (m), 1165 (w), 1087 (w), 999 (w), 819 (m), 767 (w), 748 (m), 718 (w), 680 (w), 615 (w), 543 (w).Synthesis of complex 2
A procedure similar to that used for the preparation of 1 was employed. Yield: (159 mg, 50%). Anal. Calcd for C15H17Cl3N5S2Au (632.97): C, 28.44; H, 2.71; N, 11.06. Found: C, 28.56; H, 2.88; N, 11.00. 1H NMR (500 MHz, CD3OD) δ 8.53 (t, pyridine, 1H), 8.27 (d, J = 2 Hz, imidazole, 2H), 8.10 (d, J = 2 Hz, imidazole, 2H), 8.01 (d, pyridine, 2H), 4.56–4.74 (m, 2H, CH2), 1.60 (t, 6H). 13C NMR (500 MHz, CD3OD) δ 147.71, 144.49, 143.74, 124.16, 123.89, 121.28, 46.32, 14.33. MS (MALDI-TOF): calcd for C15H17N5S2Au3+ 528.0591 (M3+), found 528.2131 (M3+). IR (KBr cm−1): 3410 (m), 3127 (w), 3087 (w), 2978 (w), 2935 (w), 1597 (s), 1473 (s), 1455 (s), 1381 (w), 1347 (w), 1313 (w), 1270 (m), 1222 (m), 1167 (w), 1091 (w), 1050 (w), 815 (w), 775 (w), 753 (w), 733 (w), 703 (w), 675 (w).Synthesis of complex 3
A procedure similar to that used for the preparation of 1 was employed. Yield: (199 mg, 60%). Anal. Calcd for C17H21Cl3N5S2Au (662.83): C, 30.86; H, 3.20; N, 10.59. Found: C, 30.75; H, 3.18; N, 11.06. 1H NMR (500 MHz, CD3OD) δ 8.53 (t, pyridine, 1H), 8.29 (d, J = 2 Hz, imidazole, 2H), 8.18 (d, J = 2 Hz, imidazole, 2H), 8.01 (d, pyridine, 2H), 5.51 (m, CH, 2H), 1.74 (d, CH3, 6H), 1.51 (d, CH3, 6H). 13C NMR (500 MHz, CD3OD) δ 147.65, 144.45, 142.90, 124.54, 121.38, 53.61, 22.70, 21.17. MS (MALDI-TOF): calcd for C17H21N5S2Au3+ 556.0904 (M3+), found 556.3727 (M3+). IR (KBr cm−1): 3408 (m), 3033 (w), 2976 (w), 1598 (m), 1550 (w), 1458 (s), 1404 (w), 1375 (w), 1345 (w), 1312 (w), 1228 (m), 1171 (w), 1076 (w), 996 (w), 960 (w), 881 (w), 811 (w), 755 (w), 729 (w), 695 (w), 633 (w).Catalytic reduction of 4-NP
The catalytic reduction of 4-NP to 4-AP was carried out at room temperature on a quartz optical cell by monitoring the electronic spectra at 1 min intervals. The degradation of 4-NP was monitored by the absorbance decrease of the electronic band at λ = 400 nm due to nitrophenolate ion in basic media and development of a new electronic band at λ = 300 nm corresponding to the formation of 4-AP. A stock solution of 4-NP (3 × 10−4 mol L−1) was prepared. For the study of reduction kinetics, 1 mL of 4-NP stock solution were transferred to the UV-Vis cell and 1.13 mg of NaBH4 (1 mL, 3 × 10−5 mol) were added. Upon addition of the reducing agent, the band at λ = 400 nm remained unaltered until addition of the gold catalyst (1 mL, 3 × 10−8 mol). In this case, the electronic spectra of the reaction mixtures were acquired at each 1 min by withdrawing 3 mL aliquots from the reaction medium.X-ray structure determination
Diffraction data of 3 was collected on a Bruker AXS SMART APEX diffractometer, equipped with a CCD area detector using Mo Kα radiation (λ = 0.71073 Å). All the data were collected at 298 K and the structures were solved by direct methods and subsequently refined on F2 by using full-matrix least-squares techniques (SHELXL),23 SADABS24 absorption corrections were applied to the data, all non-hydrogen atoms were refined anisotropically, and hydrogen atoms were located at calculated positions. All calculations were performed using the Bruker Smart program.Acknowledgements
We acknowledges financial support from the National Nature Science Foundation of China (21102004), and the Natural Science Foundation of the Anhui Higher Education Institutions of China (KJ2011A146), the Project-sponsored by SRF for ROCS and Special and Excellent Research Fund of Anhui Normal University, National Training Programs of Innovation and Entrepreneurship for Undergraduates (201410370040). Training Programs of Innovation and Entrepreneurship of Anhui Province for Undergraduates (AH201410370040).Notes and references
- (a) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed; (b) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed; (c) J. Zeng, Q. Zhang, J. Chen and Y. Xia, Nano Lett., 2009, 10, 30–35 CrossRef PubMed; (d) J. Oliver-Meseguer, J. R. Cabrero-Antonino, I. Dominguez, A. Leyva-Perez and A. Corma, Science, 2012, 338, 1452–1455 CrossRef CAS PubMed; (e) C. Della Pina, E. Falletta, L. Prati and M. Rossi, Chem. Soc. Rev., 2008, 37, 2077–2095 RSC; (f) Y.-F. Han, G.-X. Jin and F. E. Hahn, J. Am. Chem. Soc., 2013, 135, 9263–9266 CrossRef CAS PubMed.
- A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096–2126 RSC.
- (a) W. Wang, A. Zheng, P. Zhao, C. Xia and F. Li, ACS Catal., 2013, 4, 321–327 CrossRef; (b) S. Wu, J. Dzubiella, J. Kaiser, M. Drechsler, X. Guo, M. Ballauff and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 2229–2233 CrossRef CAS PubMed.
- J. Li, C.-Y. Liu and Y. Liu, J. Mater. Chem., 2012, 22, 8426–8430 RSC.
- V. A. Solovyeva, K. B. Vu, Z. Merican, R. Sougrat and V. O. Rodionov, ACS Comb. Sci., 2014, 16, 513–517 CrossRef CAS PubMed.
- X. Lv, Y. Zhu, H. Jiang, H. Zhong, X. Yang and C. Li, Dalton Trans., 2014, 43, 15111–15118 RSC.
- M. Rocha, C. Fernandes, C. Pereira, S. L. H. Rebelo, M. F. R. Pereira and C. Freire, RSC Adv., 2015, 5, 5131–5141 RSC.
- (a) J. Xie, C. Pan, A. Abdukader and C. Zhu, Chem. Soc. Rev., 2014, 43, 5245–5256 RSC; (b) T. C. Boorman and I. Larrosa, Chem. Soc. Rev., 2011, 40, 1910–1925 RSC.
- (a) W.-G. Jia, Y.-B. Huang, Y.-J. Lin and G.-X. Jin, Dalton Trans., 2008, 5612–5620 RSC; (b) W.-G. Jia, Y.-B. Huang and G.-X. Jin, J. Organomet. Chem., 2009, 694, 3376–3380 CrossRef CAS PubMed.
- W.-G. Jia, Y.-B. Huang and G.-X. Jin, J. Organomet. Chem., 2009, 694, 4008–4013 CrossRef CAS PubMed.
- Y.-F. Han, L. Zhang, L.-H. Weng and G.-X. Jin, J. Am. Chem. Soc., 2014, 136, 14608–14615 CrossRef CAS PubMed.
- J. Cordón, G. Jiménez-Osés, J. M. López-de-Luzuriaga, M. Monge, M. E. Olmos and D. Pascual, Organometallics, 2014, 33, 3823–3830 CrossRef.
- (a) G. Yuan, Y. Huo, X. Nie, H. Jiang, B. Liu, X. Fang and F. Zhao, Dalton Trans., 2013, 42, 2921–2929 RSC; (b) G. Yuan, L. Rong, X. Qiao, L. Ma and X. Wei, CrystEngComm, 2013, 15, 7307–7314 RSC; (c) W.-G. Jia, D.-D. Li, L.-Q. Yan, E.-H. Sheng and Y.-C. Dai, J. Coord. Chem., 2014, 67, 363–371 CrossRef CAS; (d) Y.-F. Han, Y. Fei and G.-X. Jin, Dalton Trans., 2010, 39, 3976–3984 RSC; (e) Y.-F. Han and G.-X. Jin, Chem. Soc. Rev., 2014, 43, 2799–2823 RSC; (f) Y.-F. Han, Y.-J. Lin and G.-X. Jin, Dalton Trans., 2011, 40, 10370–10375 RSC; (g) Y.-F. Han, H. Li and G.-X. Jin, Chem. Commun., 2010, 46, 6879–6890 RSC; (h) Y.-F. Han, H. Li, Y. Fei, Y.-J. Lin, W.-Z. Zhang and G.-X. Jin, Dalton Trans., 2010, 39, 7119–7124 RSC.
- P. Herves, M. Perez-Lorenzo, L. M. Liz-Marzan, J. Dzubiella, Y. Lu and M. Ballauff, Chem. Soc. Rev., 2012, 41, 5577–5587 RSC.
- M. J. Vaidya, S. M. Kulkarni and R. V. Chaudhari, Org. Process Res. Dev., 2003, 7, 202–208 CrossRef CAS.
- P. Pachfule, S. Kandambeth, D. Diaz Diaz and R. Banerjee, Chem. Commun., 2014, 50, 3169–3172 RSC.
- K. Kuroda, T. Ishida and M. Haruta, J. Mol. Catal. A: Chem., 2009, 298, 7–11 CrossRef CAS PubMed.
- Y. Deng, Y. Cai, Z. Sun, J. Liu, C. Liu, J. Wei, W. Li, C. Liu, Y. Wang and D. Zhao, J. Am. Chem. Soc., 2010, 132, 8466–8473 CrossRef CAS PubMed.
- H. Woo and K. H. Park, Catal. Commun., 2014, 46, 133–137 CrossRef CAS PubMed.
- Z. Zhang, C. Shao, P. Zou, P. Zhang, M. Zhang, J. Mu, Z. Guo, X. Li, C. Wang and Y. Liu, Chem. Commun., 2011, 47, 3906–3908 RSC.
- J. Zhang, D. Han, H. Zhang, M. Chaker, Y. Zhao and D. Ma, Chem. Commun., 2012, 48, 11510–11512 RSC.
- Z. Wang, H. Fu, D. Han and F. Gu, J. Mater. Chem. A, 2014, 2, 20374–20381 CAS.
- G. Sheldrick, SHELXL-97, a program for refining crystal structures, University of Göttingen, Germany, 1997 Search PubMed.
- G. Sheldrick, SADABS v. 2.01, Bruker/Siemens area detector absorption correction program, Bruker AXS, Madison, WI, USA, 1998 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic information files (CIFs) for complex 3 are available. CCDC 1044349. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra01749a |
| This journal is © The Royal Society of Chemistry 2015 |