Use of a tin antimony alloy-filled porous carbon nanofiber composite as an anode in sodium-ion batteries
Chen Chena,
Kun Fua,
Yao Lua,
Jiadeng Zhua,
Leigang Xueb,
Yi Hua and
Xiangwu Zhang*a
aNorth Carolina State University, USA. E-mail: xiangwu_zhang@ncsu.edu
bArizona State University, USA
First published on 17th March 2015
Abstract
Lithium-ion battery is currently the dominant energy storage technology for electronic devices and electric vehicles. However, the predictable rising cost of lithium raw materials has attracted increasing interest in less expensive rivals, such as sodium-ion battery. In this work, a tin antimony (SnSb) alloy-filled porous carbon nanofiber composite was prepared as a sodium-ion battery anode material by a simple electrospinning method with subsequent thermal treatment. The spinning solution contained antimony tin oxide nanoparticles as the SnSb alloy precursor, polyacrylonitrile as the carbon precursor, and polymethyl methacrylate (PMMA) as the pore generator. The resultant SnSb@C nanofiber composite formed a continuous conductive network, which was favorable for enhancing its electrochemical performance. The presence of the SnSb alloy significantly increased the energy storage capacity of the composite due to its high theoretical capacity. The porous structure created by the decomposition of the PMMA polymer provided a free space to buffer the volume change of the SnSb alloy during the sodiation–desodiation process. The resultant SnSb@C nanofiber composite exhibited high capacity and a stable rate capability, and it was demonstrated to be a promising anode candidate for sodium-ion batteries.
Introduction
Since Sony produced the first commercial lithium-ion battery in the early 1990's, the characteristics of high energy density, no memory effect, and long lifespan make lithium-ion batteries the most widely used energy storage system in various applications such as portable electronics, including laptops, cell-phones, electric vehicles and hybrid electric vehicles.1,2 However, considering the limited lithium source on earth and continuously increasing energy demand, other feasible energy storage technologies must be developed to resolve the foreseeable cost issue of lithium-ion batteries.3 As an alternative material, sodium possesses similar chemical properties to lithium, but has lower cost and higher abundance. Therefore, using the same mechanism and similar material structures, low-cost sodium-ion batteries can be developed for large-scale applications.4To date, sodium-ion battery research has been mainly focused on cathode materials including NaVPO4F,5 Na0.44MnO2,6 and Na0.85Li0.17Ni0.21Mn0.64O2.7 Compared with the development of cathode materials, fewer studies have been carried out to develop new anode materials. Graphite, a commercial anode material in lithium-ion batteries, was first studied for its use as a sodium-ion battery anode, but it was found that it is difficult for sodium ions to intercalate into the basal planes of graphite.8,9 Other carbonaceous materials have also been studied as sodium-ion battery anodes. For example, the nitrogen-doped porous carbon fibers prepared by Yan et al.10 showed a reversible capacity of around 230 mA h g−1 with good rate performance, and a template carbon produced by Philipp et al.11 exhibited a reversible capacity of around 140 mA h g−1 in 40 cycles. One major disadvantage of these carbonaceous anode materials are their relatively low capacities. Tin (Sn) and antimony (Sb) based materials have been widely investigated as lithium-ion battery anodes due to their high theoretical capacities (namely, 993 mA h g−1 for Sn and 660 mA h g−1 for Sb).12–15 Moreover, it has been observed that sodium ions are able to intercalate with Sn and Sb to form sodium alloys in a similar way to lithium alloys, producing theoretical capacities of 847 mA h g−1 (Na15Sn4) and 660 mA h g−1 (Na3Sb) for Sn and Sb, respectively.16 For example, Takayuki Yamamoto et al.17 prepared Sn electrodes and achieved a high initial capacity of 790 mA h g−1. However, the capacity reduced rapidly to only 150 mA h g−1 after 30 cycles. In lithium-ion batteries, the rapid capacity decay of Sn and Sb based anodes is typically ascribed to the large volume changes of Sn and Sb particles during the lithiation–delithiation process. Such volume changes lead to the pulverization of electrodes, which in turn causes the breakdown of the electrically conductive network and insulation of the active material.18 Because sodium ions have larger radius than lithium ions, even larger volume changes can be predicted in the sodiation–desodiation process when Sn and Sb are used as the anode materials in sodium-ion batteries. Therefore, the technological impact of developing alloy-based sodium-ion anode materials with stable structure and excellent cycling behavior would be significant and needs further exploration.
In this article, we report a Sn/Sb alloy nanoparticle-filled porous carbon (SnSb@C) nanofiber composite produced by an inexpensive electrospinning approach with subsequent thermal treatment. Fig. 1 schematically illustrates the preparation procedure of SnSb@C nanofiber composite. Electrospun composite nanofibers were first prepared using a mixed solution, containing antimony tin oxide (ATO) nanoparticles as the SnSb alloy precursor, polyacrylonitrile (PAN) as the carbon precursor, and polymethyl methacrylate (PMMA) as the pore generator. These electrospun precursor nanofibers were then thermally treated to form the SnSb@C nanofiber composite structure, during which ATO was reduced to a SnSb alloy, PAN was converted to a carbon matrix, and PMMA was completely decomposed creating a porous architecture inside the nanofibers. The electrochemical performance, including specific capacity, cycling stability, and rate capability, of the SnSb@C nanofiber composite was evaluated by galvanostatic charge–discharge tests. The results demonstrated that the carbon nanofiber matrix and the porous architecture work synthetically to buffer the volume change of the SnSb alloy during the sodiation–desodiation process, leading to high capacity, good cycling performance, and high rate capability of the SnSb@C nanofiber composite anode.
Experimental
Chemicals
Polyacrylonitrile (PAN, average Mw = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aldrich), polymethyl methacrylate (PMMA, average Mw = 120
000, Aldrich), polymethyl methacrylate (PMMA, average Mw = 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Aldrich), antimony tin oxide (SnO2/Sb2O5, ATO, particle diameter < 50 nm, Aldrich), N,N-dimethylformamide (DMF, 99.8%, Aldrich), dimethyl carbonate (DMC, ≥99%, Aldrich), ethylene carbonate (EC, 98%, Aldrich), sodium (Na, Aldrich), sodium perchlorate (NaClO4, 98%, Aldrich) were purchased from Sigma-Aldrich Chemical Company (USA) and were used without further purification.
000, Aldrich), antimony tin oxide (SnO2/Sb2O5, ATO, particle diameter < 50 nm, Aldrich), N,N-dimethylformamide (DMF, 99.8%, Aldrich), dimethyl carbonate (DMC, ≥99%, Aldrich), ethylene carbonate (EC, 98%, Aldrich), sodium (Na, Aldrich), sodium perchlorate (NaClO4, 98%, Aldrich) were purchased from Sigma-Aldrich Chemical Company (USA) and were used without further purification.
Nanofiber preparation
PAN solution (8%) in DMF was prepared by vigorous mechanical stirring for 5 h at 60 °C. ATO nanoparticles (ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN = 1
PAN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 0
1, and 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight) and PMMA polymer (PMMA
1 by weight) and PMMA polymer (PMMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN = 1
PAN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 by weight) were added into the PAN solution and vigorously stirred for 24 h at room temperature to obtain homogeneous dispersions for electrospinning.
10 by weight) were added into the PAN solution and vigorously stirred for 24 h at room temperature to obtain homogeneous dispersions for electrospinning.
Precursor nanofibers were prepared by electrospinning with an applied voltage of 15 kV, a solution flow rate of 0.75 mL h−1, and a needle tip-to-collector distance of 15 cm. To form SnSb@C nanofiber composites, electrospun precursor nanofibers were first stabilized in air at 280 °C for 5.5 h with a heating rate of 5 °C min−1, and then carbonized at 700 °C in argon for 3 h with a heating rate of 2 °C min−1. During this process, PAN was converted to form a carbon nanofiber matrix and ATO was reduced to SnSb alloy nanoparticles, while PMMA was decomposed completely, resulting in the formation of a porous structure within the carbon matrix.
Structural characterization
The XRD analysis was conducted using a Rigaku SmartLab X-ray diffractometer with Cu Kα radiation between 2θ angles from 20° to 70°. Field-emission scanning electron microscopy (FESEM, JEOL 6400) and field-emission transmission electron microscopy (FETEM, Hitachi HF2000) were employed to characterize the morphology of the precursor nanofibers and SnSb@C nanofiber composites. Thermal gravimetric analysis (Perkin Elmer Pyris 1 TGA) and CHN elemental analysis were conducted to examine the compositions of SnSb@C nanofiber composites.Electrochemical evaluation
SnSb@C nanofibers (80 wt%) were ground into a powder form and mixed with carbon black (10 wt%) and alginic acid sodium salt (10 wt%) to form a homogeneous slurry with deionized water as the solvent. The slurry was then pasted onto a copper foil, followed by drying in a vacuum oven for 24 hours. CR2032-type coin cells were assembled in an argon-filled glove box using sodium metal as the counter electrode and a microporous glass fiber membrane (Whatman) as the separator. The electrolyte used in this study was 1 M NaClO4 in a solvent of ethylene carbonate and diethyl carbonate (EC/DEC, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume).
1 by volume).
Cyclic voltammetry (CV) measurements were performed by Gamry Reference 600 Potentiostat/Galvanostat/ZRA system in a voltage range of 2.5–0.01 V with a scan rate of 0.05 mV s−1. Galvanostatic charge–discharge tests were conducted using a LAND CT2001A battery testing system in a voltage range of 0.01–2.5 V. The capacity values were calculated based on the total composite weight.
Results and discussion
Structure characterization
Fig. 2 shows the SEM images of electrospun precursor nanofibers with an ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN weight ratio of 0.5
PAN weight ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It can be seen that the as-spun precursor nanofibers are continuous and have relatively uniform diameters. A few knot-like structures are detected from the surface of the nanofibers, which can be attributed to the agglomeration of ATO nanoparticles.
1. It can be seen that the as-spun precursor nanofibers are continuous and have relatively uniform diameters. A few knot-like structures are detected from the surface of the nanofibers, which can be attributed to the agglomeration of ATO nanoparticles.
Fig. 3 shows SEM images of SnSb@C nanofibers carbonized from precursor with an ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Similar to the precursor nanofibers, SnSb@C nanofibers also form a three-dimensional network structure. A few knots, representing the aggregation of the nanoparticles, can still be observed. TEM images of these SnSb@C nanofibers are shown in Fig. 4.
1. Similar to the precursor nanofibers, SnSb@C nanofibers also form a three-dimensional network structure. A few knots, representing the aggregation of the nanoparticles, can still be observed. TEM images of these SnSb@C nanofibers are shown in Fig. 4.
SnSb nanoparticles formed in situ during carbonization are nano-sized and can facilitate a shorter lithium ion diffusion path length. These SnSb nanoparticles are encapsulated inside the carbon nanofibers. Encapsulating high-capacity active materials into a porous carbon matrix is a common practice for accommodating their large volume changes for the purpose of improving the cycling performance.19–21 For example, Yan et al. produced Sn-encapsulated porous carbon nanofiber composites by single-nozzle electrospinning and the material exhibited excellent reversible capacities, cycling performance, and rate capability due to its multichannel and porous structure.19 It can also be seen from Fig. 4 that a porous structure has been formed around the SnSb nanoparticles inside the carbon nanofiber matrix, which mainly results from the decomposition of PMMA. The porous structure can help buffer the large volume changes of SnSb nanoparticles during the sodiation–desodiation process and prevents the fracture of the carbon nanofiber matrix.
XRD patterns of ATO precursor nanoparticles and SnSb@C nanofibers are shown in Fig. 5. In Fig. 5A, characteristic peaks are observed at 26.4°, 33.8°, 38.0°, 39.0°, 51.7° 61.9°, 64.8°, and 66.0° for ATO nanoparticles. In Fig. 5B, the peaks at 30.7°, 32.0°, 44.1° and 45.1° can be attributed to the metallic Sn phase, whereas the peaks at 29.1°, 41.5°, 41.7°, 51.2°, and 60.2° can be ascribed to the SnSb phase, indicating the reduction of the ATO nanoparticles and the formation of Sn and SnSb phases. Due to the small content of Sb2O5 in the ATO precursor (7–11%), the SnSb phase shows weak peaks in the XRD pattern. Therefore, during the thermal treatment, the ATO nanoparticles were transformed to SnSb nanoparticles, consisting of Sn and SnSb phases, using a pyrolytic carbon as a reduction agent under an inert argon atmosphere.
 | ||
Fig. 5 XRD patterns of (A) ATO nanoparticles, and (B) SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5 PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
To determine the carbon content in the composite, TGA analysis was conducted in air (Fig. 6) and the result showed a carbon content of 40% for SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An elemental analysis was also conducted and the following composition was determined for the SnSn@C nanofiber composite prepared with ATO
1. An elemental analysis was also conducted and the following composition was determined for the SnSn@C nanofiber composite prepared with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1: 52.8% SnSb, 41.1% carbon, 1.0% hydrogen, and 5.1% nitrogen.
1: 52.8% SnSb, 41.1% carbon, 1.0% hydrogen, and 5.1% nitrogen.
Fig. 7 compares the Raman spectra of carbon nanofibers and SnSb@C nanofibers (ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN = 0.5
PAN = 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For both nanofibers, there are two distinct peaks at 1600 (G band) and 1350 cm−1 (D band), which are associated with the vibration of sp2 bonded carbon in planar sheets and the presence of defects and disordered carbon, respectively. The intensity ratios of the D band and G band, i.e., ID/IG ratio, is often used to assess the disorder feature of carbon materials. From Fig. 7, the ID/IG ratios of carbon nanofibers and SnSb@C nanofibers can be calculated as 1.18 and 1.19, respectively. The relatively high ID/IG ratios suggest the disordered nature of the carbon structure in both carbon nanofibers and SnSb@C nanofibers.
1). For both nanofibers, there are two distinct peaks at 1600 (G band) and 1350 cm−1 (D band), which are associated with the vibration of sp2 bonded carbon in planar sheets and the presence of defects and disordered carbon, respectively. The intensity ratios of the D band and G band, i.e., ID/IG ratio, is often used to assess the disorder feature of carbon materials. From Fig. 7, the ID/IG ratios of carbon nanofibers and SnSb@C nanofibers can be calculated as 1.18 and 1.19, respectively. The relatively high ID/IG ratios suggest the disordered nature of the carbon structure in both carbon nanofibers and SnSb@C nanofibers.
 | ||
Fig. 7 Raman spectra of (A) carbon nanofibers, and (B) SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5 PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
Electrochemical evaluation
Fig. 8 shows the cyclic voltammetry (CV) test results of SnSb@C nanofibers with different ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratios. In Fig. 8A, the electrochemical behavior of pure carbon nanofibers (i.e., ATO
PAN ratios. In Fig. 8A, the electrochemical behavior of pure carbon nanofibers (i.e., ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN = 0
PAN = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is shown. In the first reduction scan, the peak shown at 0.5 V is due to the formation of a solid electrolyte interface (SEI) on the surface of the carbon nanofibers. The sodiation process in the carbon nanofibers mainly occurs at 0.02 V, which corresponds to the peak in Fig. 8A. For SnSb@C nanofibers prepared from precursor with ATO
1) is shown. In the first reduction scan, the peak shown at 0.5 V is due to the formation of a solid electrolyte interface (SEI) on the surface of the carbon nanofibers. The sodiation process in the carbon nanofibers mainly occurs at 0.02 V, which corresponds to the peak in Fig. 8A. For SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the peak at 0.35 V in the first cycle can be mainly ascribed to the irreversible reaction that produces the SEI film.
1, the peak at 0.35 V in the first cycle can be mainly ascribed to the irreversible reaction that produces the SEI film.
 | ||
Fig. 8 Cyclic voltammetry curves of SnSb@C nanofibers prepared from precursor with different ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratios: (A) 0 PAN ratios: (A) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (B) 0.5 1, (B) 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (C) 1 1, (C) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
After the first cycle, the reductive peak at 0.02 V can be assigned to the Na-ion insertion into the carbon matrix, while that at 0.5 V is for the Na-ion insertion into the SnSb nanoparticles to form Na15Sn4 and Na3Sb. Correspondingly, the oxidative peaks at 0.1 V, 0.5 V and 0.6 V indicate the Na-ion extraction from the carbon matrix, Na15Sn4 phase, and Na3Sb phase.16 With the ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio increased to 1
PAN ratio increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the electrochemical behavior of the electrode does not change and the oxidative peaks at 0.1 V, 0.5 V and 0.6 V and reductive peaks at 0.02 V and 0.5 V can still be seen in the CV curve.
1, the electrochemical behavior of the electrode does not change and the oxidative peaks at 0.1 V, 0.5 V and 0.6 V and reductive peaks at 0.02 V and 0.5 V can still be seen in the CV curve.
Fig. 9 shows the charge–discharge curves of SnSb@C nanofibers prepared from precursors with different ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratios. The current density used was 500 mA g−1. For the pure carbon nanofibers (ATO
PAN ratios. The current density used was 500 mA g−1. For the pure carbon nanofibers (ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN = 0
PAN = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), an initial capacity of 380 mA h g−1 and reversible capacity of 161 mA h g−1 was obtained, and during the first cycle, a discharge plateau at 0.5 V can be observed, which relates to the SEI formation and corresponds to the peak observed in the CV curve (Fig. 8). The initial capacity loss of carbon nanofibers is large due to the formation of the SEI film and the irreversible reactions between sodium and surface functional groups. The large initial capacity loss leads to a low coulombic efficiency for carbon nanofibers in the initial cycle.10,22 The addition of ATO reduces the content of carbon in the entire electrode, which might lead to reduced initial capacity loss and increased coulombic efficiency. From Fig. 9, it can be seen that for SnSb@C nanofibers prepared from precursor with ATO
1), an initial capacity of 380 mA h g−1 and reversible capacity of 161 mA h g−1 was obtained, and during the first cycle, a discharge plateau at 0.5 V can be observed, which relates to the SEI formation and corresponds to the peak observed in the CV curve (Fig. 8). The initial capacity loss of carbon nanofibers is large due to the formation of the SEI film and the irreversible reactions between sodium and surface functional groups. The large initial capacity loss leads to a low coulombic efficiency for carbon nanofibers in the initial cycle.10,22 The addition of ATO reduces the content of carbon in the entire electrode, which might lead to reduced initial capacity loss and increased coulombic efficiency. From Fig. 9, it can be seen that for SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the specific discharge capacity at the first cycle is 470 mA h g−1 with a coulombic efficiency of 82.6%. The irreversible capacity loss in the first cycle is mainly attributed to the reduction of the electrolyte due to SEI formation on the surface of the electrode during the first discharge step.23 This high coulombic efficiency can be attributed to the proper size of the nanoparticles and the efficient protection of nanoparticles by the carbon nanofiber matrix. It is known that smaller particles have larger surface area, which promotes the formation of the SEI leading to a lower coulombic efficiency during the first charge–discharge process. The size of the obtained Sn and SnSb alloy nanoparticles is around 50 nm, which is beneficial for the formation of SEI and increase of the first coulombic efficiency.24,25 For the SnSb@C nanofibers prepared from precursor with ATO
1, the specific discharge capacity at the first cycle is 470 mA h g−1 with a coulombic efficiency of 82.6%. The irreversible capacity loss in the first cycle is mainly attributed to the reduction of the electrolyte due to SEI formation on the surface of the electrode during the first discharge step.23 This high coulombic efficiency can be attributed to the proper size of the nanoparticles and the efficient protection of nanoparticles by the carbon nanofiber matrix. It is known that smaller particles have larger surface area, which promotes the formation of the SEI leading to a lower coulombic efficiency during the first charge–discharge process. The size of the obtained Sn and SnSb alloy nanoparticles is around 50 nm, which is beneficial for the formation of SEI and increase of the first coulombic efficiency.24,25 For the SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 1
PAN ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the specific discharge capacity is 859 mA h g−1 at the first cycle and the coulombic efficiency is 76.0%. The initial capacity loss of SnSb@C nanofibers prepared with a lower ATO
1, the specific discharge capacity is 859 mA h g−1 at the first cycle and the coulombic efficiency is 76.0%. The initial capacity loss of SnSb@C nanofibers prepared with a lower ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is considerably smaller than that of SnSb@C nanofibers prepared with a higher ATO
1 is considerably smaller than that of SnSb@C nanofibers prepared with a higher ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 1
PAN ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. From the subsequent discharge curves, the plateau at 0.5 V indicates the insertion of Na ion into SnSb nanoparticles, which corresponds to the peak at 0.5 V in the CV curve. After the 2nd cycle, the specific capacity tends to stabilize and the coulombic efficiency increases to above 95.0%.
1. From the subsequent discharge curves, the plateau at 0.5 V indicates the insertion of Na ion into SnSb nanoparticles, which corresponds to the peak at 0.5 V in the CV curve. After the 2nd cycle, the specific capacity tends to stabilize and the coulombic efficiency increases to above 95.0%.
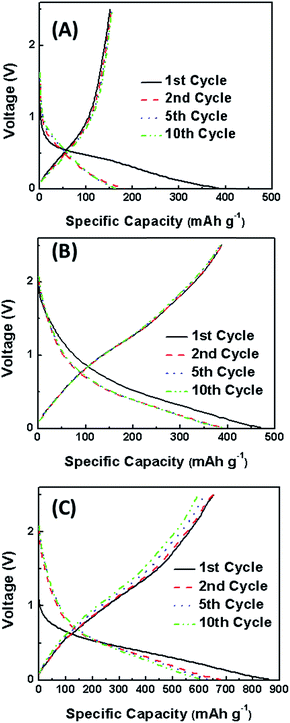 | ||
Fig. 9 Charge–discharge curves of SnSb@C nanofibers prepared from precursors with different ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratios: (A) 0 PAN ratios: (A) 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (B) 0.5 1, (B) 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (C) 1 1, (C) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The cycling performance of SnSb@C nanofibers prepared from precursors with different ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratios were also evaluated at a current density of 500 mA g−1 and the results are shown in Fig. 10. Pure PAN-derived carbon nanofibers (ATO
PAN ratios were also evaluated at a current density of 500 mA g−1 and the results are shown in Fig. 10. Pure PAN-derived carbon nanofibers (ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN = 0
PAN = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) show a capacity of 130 mA h g−1 in most cycles and this capacity value is significantly lower than those of SnSb@C nanofibers prepared with ATO
1) show a capacity of 130 mA h g−1 in most cycles and this capacity value is significantly lower than those of SnSb@C nanofibers prepared with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratios of 0.5
PAN ratios of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This indicates that the majority of the capacity of SnSb@C nanoparticles is provided by SnSb nanoparticles. From Fig. 10, it is seen that when the ATO
1. This indicates that the majority of the capacity of SnSb@C nanoparticles is provided by SnSb nanoparticles. From Fig. 10, it is seen that when the ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN is 0.5
PAN is 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a reversible capacity of 380 mA h g−1 is achieved after the first cycle, and the capacity gradually decreases to 356 mA h g−1 at the 200th charge–discharge cycle, indicating a capacity retention of as high as 93.7%. When the ATO
1, a reversible capacity of 380 mA h g−1 is achieved after the first cycle, and the capacity gradually decreases to 356 mA h g−1 at the 200th charge–discharge cycle, indicating a capacity retention of as high as 93.7%. When the ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio is 1
PAN ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a high initial capacity is expected due to the presence of higher amount of SnSb nanoparticles. A high reversible capacity of 649 mA h g−1 is achieved after the first cycle; however, the capacity decreases to 410 mA h g−1 at the 200th cycle, indicating a capacity retention of 63.2%. Therefore, the capacity retention of SnSb@C nanofibers prepared with ATO
1, a high initial capacity is expected due to the presence of higher amount of SnSb nanoparticles. A high reversible capacity of 649 mA h g−1 is achieved after the first cycle; however, the capacity decreases to 410 mA h g−1 at the 200th cycle, indicating a capacity retention of 63.2%. Therefore, the capacity retention of SnSb@C nanofibers prepared with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 1
PAN ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is lower than that of SnSb@C nanofibers with ATO
1 is lower than that of SnSb@C nanofibers with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This is because in SnSb@C nanofibers prepared with ATO
1. This is because in SnSb@C nanofibers prepared with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 1
PAN ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, some SnSb nanoparticles may not be completely encapsulated inside the carbon nanofiber matrix and the volume change of unprotected SnSb nanoparticles is not effectively buffered during the sodiation–desodiation process, resulting in a larger capacity loss. The excellent cycling behavior of the SnSb@C nanofibers prepared with ATO
1, some SnSb nanoparticles may not be completely encapsulated inside the carbon nanofiber matrix and the volume change of unprotected SnSb nanoparticles is not effectively buffered during the sodiation–desodiation process, resulting in a larger capacity loss. The excellent cycling behavior of the SnSb@C nanofibers prepared with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be attributed to both the buffering effect of the carbon nanofiber matrix and the void space surrounding the SnSb nanoparticles.
1 can be attributed to both the buffering effect of the carbon nanofiber matrix and the void space surrounding the SnSb nanoparticles.
 | ||
| Fig. 10 Cycling performance of SnSb@C nanofibers prepared from precursors with different ATO/PAN ratios under current density of 500 mA g−1. | ||
The rate capability of SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5
PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was studied by increasing the current density sequentially from 50 mA g−1 to 100 mA g−1, 200 mA g−1, 500 mA g−1, and 1 A g−1 (Fig. 11).
1 was studied by increasing the current density sequentially from 50 mA g−1 to 100 mA g−1, 200 mA g−1, 500 mA g−1, and 1 A g−1 (Fig. 11).
 | ||
Fig. 11 Rate capability of SnSb@C nanofibers prepared from precursor with ATO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PAN ratio of 0.5 PAN ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 from 50 to 1000 mA g−1. 1 from 50 to 1000 mA g−1. | ||
It is seen that at 50 mA g−1, the SnSb@C nanofibers deliver a capacity of 590 mA g−1. As the current density increases, the capacity gradually decreases; however, a relatively high capacity of 370 mA h g−1 is still maintained at 1 A g−1, corresponding to a capacity retention of 62.7% with respect to the capacity at 50 mA g−1. When the current density returns back to 50 mA g−1 after 50 cycles, a capacity as high as 560 mA h g−1 is restored, revealing a good reversibility, which confirms that the SnSb@C nanofibers have a stable structure. This excellent rate capability can be attributed to the binary alloy of SnSb, which can act as the buffer to alleviate the volume change for each other and the porous carbon nanofiber structure, which can accommodate the volume change of SnSb nanoparticles while providing conductive pathways for both electrons and Na ions.26 Moreover, the porous structure around the nanoparticles can also facilitate the diffusion of the electrolyte and Na ions, which promotes the kinetics of the electrode reactions.
To investigate the morphology change of SnSb@C nanofibers, the cell was disassembled and examined by TEM after 200 charge–discharge cycles (Fig. 12). It can be seen that the spherulitic structure of SnSb nanoparticles was maintained and that most particles were still encapsulated in the carbon nanofiber matrix, suggesting that pulverization and aggregation of SnSb nanoparticles inside the carbon nanofiber matrix were effectively alleviated.
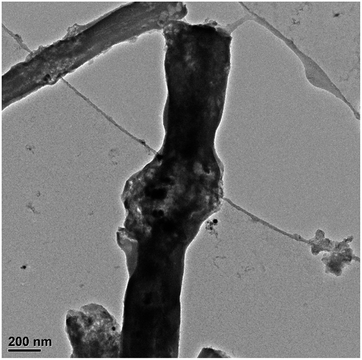 | ||
| Fig. 12 TEM image of SnSb@C nanofibers after 200 charge–discharge cycles under the current density of 500 mA g−1. | ||
Conclusion
In this work, ATO nanoparticles, PAN and PMMA were utilized to produce a SnSb nanoparticle-filled porous carbon fiber composite for use as an anode material in sodium-ion batteries. The morphology, active material content and electrochemistry performance were evaluated using XRD, SEM, TEM, and elemental analysis. The uniform and stable porous structure, confirmed by the SEM and TEM images, provided space and confinement for SnSb nanoparticles and buffered the volume expansion-contraction during repeated charge–discharge cycling. The high capacity and excellent cycling performance under high current density demonstrated the good electronic contact from carbon nanofibers during cycling and excellent stability of the active material.Acknowledgements
This research was supported by National Science Foundation under Award Number CMMI-1231287 and Zhejiang Provincial Natural Science Foundation under Award Number LY12E03005.References
- V. L. Chevrier and G. Ceder, J. Electrochem. Soc., 2011, 158, A1011 CrossRef CAS PubMed.
- Z. Wang, Z. Wang, W. Liu, W. Xiao and X. W. Lou, Energy Environ. Sci., 2013, 6, 87 CAS.
- Y. Zhu, X. Han, Y. Xu, Y. Liu, S. Zheng, K. Xu, L. Hu and C. Wang, ACS Nano, 2013, 7, 6378 CrossRef CAS PubMed.
- S.-W. Kim, D.-H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710 CrossRef CAS.
- Y. Lu, S. Zhang, Y. Li, L. Xue, G. Xu and X. Zhang, J. Power Sources, 2014, 247, 770 CrossRef CAS PubMed.
- H. Kim, D. J. Kim, D. Seo, M. S. Yeom, K. Kang and D. K. Kim, Chem. Mater., 2012, 24, 1205 CrossRef CAS.
- D. Kim, S.-H. Kang, M. Slater, S. Rood, J. T. Vaughey, N. Karan, M. Balasubramanian and C. S. Johnson, Adv. Energy Mater., 2011, 1, 333 CrossRef CAS.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2001, 148, A803 CrossRef CAS PubMed.
- Y.-M. Lin, P. R. Abel, A. Gupta, J. B. Goodenough, A. Heller and C. B. Mullins, ACS Appl. Mater. Interfaces, 2013, 5, 8273 CAS.
- L. Fu, K. Tang, K. Song, P. A. van Aken, Y. Yu and J. Maier, Nanoscale, 2014, 6, 1384 RSC.
- S. Wenzel, T. Hara, J. Janek and P. Adelhelm, Energy Environ. Sci., 2011, 4, 3342 CAS.
- L. Xue, X. Xia, T. Tucker, K. Fu, S. Zhang, S. Li and X. Zhang, J. Mater. Chem. A, 2013, 1, 13807 CAS.
- Y. Wang and J. Y. Lee, Angew. Chem., Int. Ed., 2006, 45, 7039 CrossRef CAS PubMed.
- K. T. Lee, Y. S. Jung and S. M. Oh, J. Am. Chem. Soc., 2003, 125, 5652 CrossRef CAS PubMed.
- J. Qin, C. He, N. Zhao, Z. Wang, C. Shi, E.-Z. Liu and J. Li, ACS Nano, 2014, 8, 1728 CrossRef CAS PubMed.
- L. Xiao, Y. Cao, J. Xiao, W. Wang, L. Kovarik, Z. Nie and J. Liu, Chem. Commun., 2012, 48, 3321 RSC.
- T. Yamamoto, T. Nohira, R. Hagiwara, A. Fukunaga, S. Sakai, K. Nitta and S. Inazawa, J. Power Sources, 2012, 217, 479 CrossRef CAS PubMed.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Energy Environ. Sci., 2012, 5, 5884 CAS.
- Y. Yu, L. Gu, C. Wang, A. Dhanabalan, P. a. Van Aken and J. Maier, Angew. Chem., Int. Ed., 2009, 48, 6485 CrossRef CAS PubMed.
- Y. Yu, L. Gu, C. Zhu, P. a. Van Aken and J. Maier, J. Am. Chem. Soc., 2009, 131, 15984 CrossRef CAS PubMed.
- K. Fu, L. Xue, O. Yildiz, S. Li, H. Lee, Y. Li, G. Xu, L. Zhou, P. D. Bradford and X. Zhang, Nano Energy, 2013, 2, 976 CrossRef CAS PubMed.
- Z. Wang, L. Qie, L. Yuan, W. Zhang, X. Hu and Y. Huang, Carbon, 2013, 55, 328 CrossRef CAS PubMed.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395 CrossRef CAS.
- A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef PubMed.
- A. Trifonova, Solid State Ionics, 2004, 168, 51 CrossRef CAS PubMed.
- M. Wachtler, M. Winter and J. O. Besenhard, J. Power Sources, 2002, 105, 151 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |





