Recognition of fluoride anions at low ppm level inside living cells and from fluorosis affected tooth and saliva samples†
Abstract
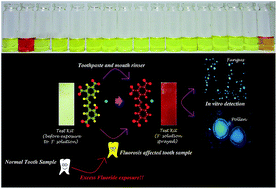
A simple Schiff base chemosensor 2-((2-(2,4-dinitro phenyl)hydrazono)methyl)-4-nitrophenol (L) has been developed as a colorimetric and fluorimetric ‘turn on’ sensor for fluoride (F−). F− recognition at ppm levels from mouth rinses and a toothpaste water solution has been successful. Significantly, L can detect F− from fluorosis affected tooth and saliva samples by similar colorimetric changes. A test kit for F− detection from a DMSO–water (1 : 1) mixture is also engineered. Intracellular F− from pollen grains of Techoma stans and Candida albicans (a diploid fungus), grown in 10−6 (M) F− contaminated water has been successfully detected under a fluorescence microscope.

 Please wait while we load your content...
Please wait while we load your content...