DOI:
10.1039/C5RA01702E
(Paper)
RSC Adv., 2015,
5, 30851-30860
Hydrothermal synthesis of bismuth oxybromide–bismuth oxyiodide composites with high visible light photocatalytic performance for the degradation of CV and phenol†
Received
28th January 2015
, Accepted 18th March 2015
First published on 18th March 2015
Abstract
This is the first report on a series of BiOpBrq/BiOmIn heterojunctions that were prepared using controlled hydrothermal methods. The compositions and morphologies of the BiOpBrq/BiOmIn could be controlled by adjusting some growth parameters, including reaction pH value, temperature, time, and KBr/KI molar ratio. The photocatalytic efficiency of the powder suspensions was evaluated by measuring crystal violet (CV) and phenol concentrations. Both the photocatalytic process and the photosensitized process were found to work concurrently. The quenching effects of various scavengers and EPR indicated that the reactive O2˙− played a major role and ˙OH and h+ played minor roles. The photocatalytic activity of the BiOpBrq/BiOmIn heterojunctons reached the maximum rate constant of 0.5285 (or 0.4713) h−1, 10 times higher than that of P25–TiO2, 14 times higher than that of BiOBr, and 6 times higher than that of BiOI.
1. Introduction
The large amount of dyes used in the dyeing stage of textile manufacturing processes represents an increasing environmental danger due to their refractory carcinogenic nature. Particularly, triphenylmethane (TPM) dyes are consumed heavily in the leather, cosmetic, paper, and food industries for the coloring of plastics, oil, fats, waxes, and varnish.1 The photocytotoxicity of TPM dyes, based on the production of reactive oxygen species, has been intensively studied with regard to photodynamic treatment.2 However, there is a great concern about the thyroid peroxidase-catalyzed oxidation of TPM dyes because the reactions might form various N-de-alkylated aromatic amines, whose structures are similar to aromatic amine carcinogens.3 Cationic TPM dyes are widely used as antimicrobial agents. Recent reports indicated that they might further serve as targetable sensitizers in the photo-destruction of specific cellular components or cells.2,3 Photocatalysis has been successfully used for degrading TPM dye pollutants in the past few years.4,5 In view of the efficient utilization of visible light, the development of new and efficient visible-light-driven photocatalysts remains a major challenge from the practical use and commercial viewpoints.
Recently, the development of visible-light-sensitive photocatalysts has received considerable attention as an alternative to wastewater treatment. An effective and simple strategy to improve the photocatalytic activity of a photocatalyst is the incorporation of a heterostructure (or composite), because heterojunctions (or composites) have great potential for tuning the desired electronic properties of composite photocatalysts and efficiently separating the photogenerated electron–hole pairs.6–12 In recent years, a new family of promising photocatalysts, bismuth oxyhalides,13–16 which belong to the V-VI-VII family of multi-component metal oxyhalides, have demonstrated remarkable photocatalytic activities due to their uniquely layered structures with an internal static electric field perpendicular to each layer, which can induce the effective separation of photogenerated electron–hole pairs. An effective and simple tactic to improve the photocatalytic activity of a photocatalyst is the architecture of the heterostructure, as the heterojunction has great potential for tuning the desired electronic properties of the composite photocatalysts and efficiently separating the photogenerated electron–hole pairs.17 Xiao et al.18 reported the synthesis of BiOI/BiOCl phases exhibiting high photocatalytic activities under visible light irradiation for the degradation of bisphenol-A, respectively. Cao et al.19 developed a facile synthesis of BiOCl/BiOI by a sol–gel process.
This research reports the preparation and characterization of a series of BiOpBrq/BiOmIn photocatalysts. This is the first report demonstrating a systematic synthesis study on BiOpBrq/BiOmIn photocatalysts by autoclave hydrothermal methods. The photocatalytic activities of the BiOpBrq/BiOmIn photocatalysts were evaluated by measuring the degradation rates of CV and phenol, and found to have excellent activity under visible light irradiation. In comparison to P25–TiO2, the new photoactive material demonstrates a higher rate for removing aqueous CV under visible light irradiation.
2. Experimental
2.1 Materials
The purchased Bi(NO3)3·5H2O, KI (Katayama), KBr (Shimakyu), CV dye (TCI), p-benzoquinone (Alfa Aesar), sodium azide (Sigma), ammonium oxalate (Osaka), and isopropanol (Merck) were obtained and used without any further purification. Reagent-grade sodium hydroxide, nitric acid, ammonium acetate and HPLC-grade methanol were obtained from Merck.
2.2 Synthesis of BiOpBrq/BiOmIn
5 mmol Bi (NO3)3·5H2O was first mixed in a 50 mL flask and then 5 mL of 4 M HNO3 was added. With continuous stirring, 2 M NaOH was added dropwise to adjust the pH value and, when a precipitate formed, 2 mL of KBr and KI were also added dropwise. The solution was then stirred vigorously for 30 min and transferred into a 30 mL Teflon-lined autoclave, which was heated up to 110–260 °C for 12 h and then naturally cooled down to room temperature. The resulting solid precipitate was collected by filtration, washed with deionized water and methanol to remove any possible ionic species in the solid precipitate, and then dried at 60 °C overnight. Depending on the molar ratio of KBr to KI (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), pH value, temperature, and time, different BiOpBrq/BiOmIn composites could be synthesized and were labeled as in Table S1 of the ESI,† namely BB1I2-1-110-12 (Br
2), pH value, temperature, and time, different BiOpBrq/BiOmIn composites could be synthesized and were labeled as in Table S1 of the ESI,† namely BB1I2-1-110-12 (Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = 1
I = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; pH = 1; temp = 110 °C; time = 12 h) to BB2I1-13-260-12 (Br
2; pH = 1; temp = 110 °C; time = 12 h) to BB2I1-13-260-12 (Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = 2
I = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; pH = 13; temp = 260 °C; time = 12 h) for the as-prepared samples, respectively.
1; pH = 13; temp = 260 °C; time = 12 h) for the as-prepared samples, respectively.
2.3 Instruments and analytical methods
The products were characterized using XRD, FE-SEM-EDS, HRXPS, FE-TEM-EDS, CL, EPR, and BET. The intermediates formed during the decomposition process were isolated, identified, and characterized using HPLC-PDA-ESI-MS. The detailed conditions are described in the ESI.†
2.4 Photocatalytic activity test
An aqueous suspension of CV (100 mL, 10 ppm) and an amount of the catalyst powder were placed in a Pyrex flask. The pH value of the suspension was adjusted by adding either NaOH or HNO3 solution. Prior to irradiation, the suspension was magnetically stirred in the dark for ca. 30 min to establish an adsorption/desorption equilibrium between the dye and the surface of the catalyst under ambient air-equilibrated conditions. The irradiation was carried out using visible-light lamps (150 W Xe arc). The light intensity was fixed at 32.1 Wm−2 when the reactor was placed 30 cm away from the light source. The irradiation experiments of CV were carried out on a stirring aqueous solution contained in a 100 mL flask. At the given irradiation time intervals, a 5 mL aliquot was collected and centrifuged to remove the catalyst. The supernatant was analyzed by HPLC-ESI-MS after readjusting the chromatographic conditions in order to make the mobile phase compatible with the working conditions of the mass spectrometer.
3. Results and discussion
3.1 Phase structure
Fig. 1 and S1–S7 (ESI†) show the XRD patterns of the as-prepared BiOpBrq/BiOmIn samples. The XRD patterns clearly reveal the coexistence of different phases. All the BiOpBrq/BiOmIn samples prepared using the described hydrothermal method at different temperatures and pH contain the BiOBr (JCPDS 09-0393), Bi4O5Br2 (JCPDS 37-0669), Bi24O31Br10 (JCPDS 75-0888), Bi3O4Br (JCPDS 84-0793), Bi5O7Br (JCPDS 38-0493), Bi12O17Br2 (JCPDS 37-0701), BiOI (JCPDS 73-2062), Bi7O9I3,20,21 and Bi5O7I (JCPDS 40-0548) phases. The XRD patterns for pH = 1, 4, 7, 10, and 13 are identical to those reported for the BiOI single phase, the BiOBr/BiOI, Bi3O4Br/Bi7O9I3, Bi5O7Br/Bi5O7I, Bi24O31Br10/BiOI, Bi3O4Br/Bi5O7I binary phase, and the BiOBr/Bi24O31Br10/BiOI, BiOBr/Bi4O5Br2/BiOI ternary phases. Table 1 summarizes the results of the XRD measurements.
 |
| | Fig. 1 XRD patterns of the as-prepared BiOpBrq/BiOmIn samples under different pH values. (Molar ratio KBr/KI = 1/2, hydrothermal conditions: temp = 110 °C, pH = 1–13, time = 12 h). | |
Table 1 Crystalline phase changes of the binary bismuth oxyhalide nanosheets prepared under different reaction conditions ( BiOBr;
BiOBr;  Bi4O5Br2;
Bi4O5Br2;  Bi24O31Br10;
Bi24O31Br10;  Bi3O4Br;
Bi3O4Br;  Bi12O17Br2;
Bi12O17Br2;  Bi5O7Br;
Bi5O7Br;  BiOI;
BiOI;  Bi7O9I3;
Bi7O9I3;  Bi5O7I)
Bi5O7I)
| pH |
Temp (°C) |
| 110 |
160 |
210 |
260 |
| BB1I2 |
| 1 |
 |
 |
 |
 |
| 4 |
 |
 |
 |
 |
| 7 |
 |
 |
 |
 |
| 10 |
 |
 |
 |
 |
| 13 |
 |
 |
 |
 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| BB2I1 |
| 1 |
 |
 |
 |
 |
| 4 |
 |
 |
 |
 |
| 7 |
 |
 |
 |
 |
| 10 |
 |
 |
 |
 |
| 13 |
 |
 |
 |
 |
Fig. 2–4 display that BB1I2-4-210-12, BB2I1-4-110-12, and BB1I2-4-110-12 are composed of sheets or plates with different sizes, consistent with the TEM observation. In addition, the EDS spectrum shows the sample contains Bi, O, Br and I elements. The TEM-EDS results show that the main elements of these samples are bismuth, iodine, chlorine, and oxygen. The Br (or I) atomic ratio (%) of the samples is within the range of 3.86–7.83 (or 9.55–15.37), corresponding to different heterojunctions. The HRTEM image reveals that two sets of different lattice images are found with the d spacing of 0.2824 and 0.3012 nm, corresponding to the (4 0 2) plane of Bi4O5Br2 and the (1 0 2) plane of BiOI (Fig. 2), 0.2814 and 0.2824 nm, corresponding to the (0 2 0) plane of Bi4O5Br2 and the (1 1 0) plane of BiOI (Fig. 3), and 0.2836 and 0.2812 nm, corresponding to the (1 2 0) plane of Bi4O5Br2 and the (1 1 0) plane of BiOI (Fig. 4), respectively, which is in good accordance with the results of the XRD patterns. The results suggest that Bi4O5Br/BiOI has formed in the composites, which will favor the separation of the photoinduced carriers and thus acquire high photocatalytic activities.
 |
| | Fig. 2 FE-TEM images and EDS of the BB1I2-4-210-12 sample prepared by the hydrothermal autoclave method. | |
 |
| | Fig. 3 FE-TEM images and EDS of the BB2I1-4-110-12 sample prepared by the hydrothermal autoclave method. | |
 |
| | Fig. 4 FE-TEM images and EDS of the BB1I2-4-110-12 sample prepared by the hydrothermal autoclave method. | |
In this experiment, the pH value plays a major role in controlling the composition and anisotropic growth of the crystals. The results show that a series of changes in the compounds occur at different pH values of the hydrothermal reactions, described as BiOBr → Bi4O5Br2 → Bi24O31Br10 → Bi3O4Br → Bi5O7Br → Bi12O17Br2 → α-Bi2O3 and BiOI → Bi4O5I2 → Bi7O9I3 → Bi3O4I → Bi5O7I → α-Bi2O3. The possible processes for the formation of the BiOpBrq/BiOmIn samples are described as follows [eqn (1)–(18)].
| | |
Bi3+ + 3OH− → Bi(OH)3(s)
| (1) |
| | |
2Bi(OH)3(s) + Br− → BiOBr(s) + H2O + OH−
| (2) |
| | |
Bi3+ + 3Br− → BiBr3(s)
| (3) |
| | |
BiBr3(s) + 2OH− → BiOBr(s) + 2Br− + H2O
| (4) |
| | |
4BiOBr(s) + 2OH− → Bi4O5Br2(s) + 2Br− + H2O
| (5) |
| | |
6Bi4O5Br2(s) + 2OH− → Bi24O31Br10(s) + 2Br− + H2O
| (6) |
| | |
Bi24O31Br10(s) + 2OH− → 8Bi3O4Br(s) + 2Br− + H2O
| (7) |
| | |
5Bi3O4Br(s) + 2OH− → 3Bi5O7Br(s) + 2Br− + H2O
| (8) |
| | |
12Bi5O7Br(s) + 2OH− → 5Bi12O17Br2 (s) + 2Br− + H2O
| (9) |
| | |
Bi12O17Br2(s) + 2OH− → 6Bi2O3(s) + 2Br− + H2O
| (10) |
| | |
2Bi(OH)3(s) +I− → BiOI(s) + H2O + OH−
| (11) |
| | |
BiI3(s) + 2OH− → BiOI(s) + 2I− + H2O
| (13) |
| | |
4BiOI(s) + 2OH− → Bi4O5I2(s) + 2I− + H2O
| (14) |
| | |
7Bi4O5I2(s) + 2OH− → 4Bi7O9I3(s) + 2I− + H2O
| (15) |
| | |
3Bi7O9I3(s) + 2OH− → 7Bi3O4I(s) + 2I− + H2O
| (16) |
| | |
5Bi3O4I(s) + 2OH− → 3Bi5O7I(s) + 2I− + H2O
| (17) |
| | |
2Bi5O7I(s) + 2OH− → 5Bi2O3(s) + 2I− + H2O
| (18) |
These equations show that BiOBr (or BiOI) is formed at the beginning of the reaction, and then OH− gradually substitutes Br− (or I−) in basic conditions, resulting in the reduced content of Br− (or I−) in the products. With increasing pH, BiOBr, Bi4O5Br2, Bi24O31Br10, Bi3O4Br, Bi5O7Br, Bi12O17Br2, and BiOI, Bi4O5I2, Bi7O9I3, Bi3O4I, Bi5O7I, and α-Bi2O3 could be obtained gradually. The higher pH value reveals the lower Br− (or I−) content in the products, until the content of Br− (or I−) in the products is fully replaced by OH−, finally resulting in the formation of α-Bi2O3 under strongly basic conditions. A competitive relationship typically exists among the OH−, Br−, and I− ions in the aqueous solution. It is demonstrated that a different BiOpBrq/BiOmIn can be selectively prepared through adjusting the pH value under the hydrothermal method.
3.2 Morphological structure and composition
The surface morphology of the BiOpBrq/BiOmIn was examined by FE-SEM-EDS (Fig. S8 and S9, ESI†). From Table 2, the EDS results show that the main elements of these samples are bismuth, bromine, iodine, and oxygen under different pH values. The detailed morphological structure and composition are described in the ESI.†
Table 2 Physical and chemical properties of BiOpBrq/BiOmIn
| Catalyst code |
EDS of atomic ratio (%) |
XPS of atomic ratio (%) |
Eg (eV) |
| Bi |
O |
Br |
I |
Bi |
O |
Br |
I |
| BB1I2-1-110-12 |
31.64 |
39.72 |
11.04 |
17.59 |
70.9 |
16.3 |
8.8 |
3.9 |
1.58 |
| BB1I2-4-110-12 |
25.35 |
54.35 |
7.52 |
12.78 |
89.2 |
5.1 |
2.4 |
3.3 |
1.80 |
| BB1I2-7-110-12 |
33.80 |
52.00 |
2.77 |
11.43 |
88.8 |
9.0 |
0.8 |
1.4 |
1.97 |
| BB1I2-10-110-12 |
31.29 |
59.85 |
1.11 |
7.75 |
85.2 |
11.9 |
0.8 |
2.1 |
2.18 |
| BB1I2-13-110-12 |
36.25 |
57.06 |
0.06 |
6.63 |
59.2 |
37.0 |
0.4 |
3.5 |
2.56 |
| BB2I1-1-110-12 |
24.03 |
48.33 |
19.21 |
8.44 |
77.8 |
12.6 |
8.4 |
1.2 |
1.68 |
| BB2I1-4-110-12 |
31.22 |
48.18 |
13.30 |
7.30 |
81.7 |
11.5 |
5.0 |
1.7 |
1.90 |
| BB2I1-7-110-12 |
30.23 |
50.52 |
12.97 |
6.27 |
79.0 |
15.4 |
4.8 |
0.9 |
1.83 |
| BB2I1-10-110-12 |
33.49 |
55.41 |
5.58 |
5.52 |
72.2 |
22.9 |
3.2 |
1.7 |
2.16 |
| BB2I1-13-110-12 |
32.80 |
58.33 |
1.23 |
7.64 |
65.9 |
31.1 |
2.4 |
0.5 |
2.38 |
| BB3-1-110-12 |
28.54 |
45.45 |
26.00 |
— |
59.5 |
22.0 |
18.5 |
— |
2.61 |
| BI3-1-110-12 |
24.47 |
50.22 |
— |
25.31 |
80.3 |
11.9 |
— |
7.8 |
1.59 |
3.3 XPS analysis
XPS was employed to examine the purity of the prepared BiOpBrq/BiOmIn products, and the spectra are shown in Fig. 5 and 6. Fig. 5 shows the total survey spectra of Bi 4f, Br 3d, I 3d, and O 1s XPS of the five BiOpBrq/BiOmIn samples. According to Fig. 5(a), the observation of transition peaks involving the Bi 4f, Br 3d, I 3d, O 1s, and C 1s orbitals reveals that the catalysts are constituted by the elements Bi, O, Br, I, and C. The characteristic binding energy value of 158.1–158.9 eV for Bi 4f7/2 (Fig. 5(b)) reveals a trivalent oxidation state for bismuth. An additional spin–orbit doublet with the binding energy of 155.7–156.3 eV for Bi 4f7/2 was also observed in all samples, suggesting that certain parts of bismuth exist in the (+3 − x) valence state. This indicates that the trivalent bismuth is partially reduced to the lower valence state by the hydrothermal autoclave method. A similar chemical shift of approximately 2.4–2.6 eV for Bi 4f7/2 was also observed by Chen et al.5 They concluded that the Bi(+3−x) formal oxidation state could most probably be attributed to the substoichiometric forms of Bi within the Bi2O2 layer and the formation of the low oxidation state resulted in oxygen vacancies in the crystal lattice. However, it was assumed that the Bi(+3−x) formal oxidation state could most likely be attributed to the substoichiometric forms of Bi at the outer site of the particles and the formation of the low oxidation state resulted in oxygen vacancies in the crystal surface. Fig. 5(c) shows the high-resolution XPS spectra for the O 1s region, which can be fitted into two peaks. The main peak at 529.1 eV is attributed to the Bi–O bonds in the (Bi2O2) 2+ slabs of the BiOX layered structure, and the peak at 530.9 eV is assigned to the hydroxyl groups on the surface.15 From Fig. 5(d), the binding energies of 67.4–67.8 eV and 68.5–68.9 eV are attributed to the Br 3d5/2 and 3d3/2 respectively which can be assigned to Br in the monovalent oxidation state. The binding energies of 617.8–618.7 eV and 629.5–630.2 eV are attributed to I 3d5/2 and 3d3/2 respectively, which can be assigned to I in the monovalent oxidation state (Fig. 5(e)). In the Bi2O3 (BB3-1-110-12 (BiOBr) or BI3-1-110-12 (BiOI)) samples, two sets of peaks centered at 164.0 and 158.5 (164.2 and 158.7 or 163.5 and 158.1) eV can be attributed to Bi 4f5/2 and Bi 4f7/2, demonstrating that the main chemical states of the bismuth element in the samples is trivalent. The characteristic binding energy reveals a trivalent oxidation state for the Bi+3–O (Bi+3–Br or Bi+3–I) bonding. Two additional spin–orbit doublets with the binding energy for Bi 4f7/2 are also observed, suggesting that certain parts of bismuth exist in the Bi+3−x–O (Bi+3−x–Br or Bi+3−x–I) bonding.5 The XPS results reveal that the possible processes for the formation of bismuth oxybromoiodides are described as eqn (1)–(18), which are consistent with the previous results by XRD and TEM analyses. Besides, the results show that the main elements of these samples are bismuth, bromine, iodine, and oxygen from Table 2. The Br (or I) atomic ratio (%) of the samples is within the range of 0.4–8.8 (or 0.5–3.9), corresponding to different BiOpBrq/BiOmIn composites.
 |
| | Fig. 5 High resolution XPS spectra of the as-prepared BiOpBrq/BiOmIn samples under different pH values. (a) total survey; (b) Bi 4f; (c) O 1s; (d) Br 3d; (e) I 3d. (Molar ratio KBr/KI = 1/2.) | |
 |
| | Fig. 6 High resolution XPS spectra of the as-prepared BiOpBrq/BiOmIn samples under different pH values. (a) total survey; (b) Bi 4f; (c) O 1s; (d) Br 3d; (e) I 3d. (Molar ratio KBr/KI = 2/1). | |
3.4 Photophysical properties of the new photocatalysts
The UV-vis diffuse reflectance spectra of the different catalysts are shown in Fig. 7(a). It can be observed that BiOBr absorbs visible light slightly while the absorption edge of BiOI extends to the whole spectrum of visible light. Moreover, the absorption edges of the BiOpBrq/BiOmIn composites have a monotonic red shift response of BiOpBrq. Based on the absorption spectra, the Eg of the semiconductor can be calculated from the αhν = A(hν − Eg)n/2 equation.19,22 The values of n for BiOBr and BiOI are 4 and 4, respectively. The Eg of BiOpBrq/BiOmIn was determined from a plot of (αhν)1/2 vs. energy (hν) in Fig. 7(b) and elicited to be 1.58–2.56 eV in Table 2. The difference of the band gap energies in the as-prepared BiOpBrq/BiOmIn can be ascribed to their individual composition with various characteristics. The steep shape and strong absorption in the visible region ascribe the visible light absorption to the intrinsic band gap transition between the valence band and the conduction band, rather than the transition from the impurity levels.22
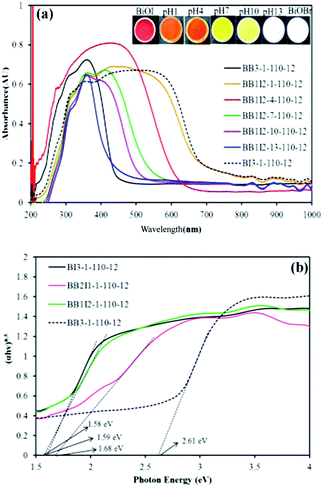 |
| | Fig. 7 UV–vis absorption spectra of the as-prepared BiOpBrq/BiOmIn samples under different (a) pH values and (b) molar ratios. | |
3.5 Specific surface areas and pore structure
Fig. S10(a) of the ESI† shows the nitrogen adsorption–desorption isotherm curves of the BiOpBrq/BiOmIn samples for different pH values. The isotherms of all the samples are close to type IV (Brunauer–Deming–Deming–Teller, BDDT, classification) with a hysteresis loop at a high relative pressure between 0.6 and 1.0.23 From Fig. S10(b) of the ESI,† the shape of the hysteresis loop is close to Type H3, suggesting the existence of slit-like pores generally formed by the aggregation of plate-like particles, which is consistent with the self-assembled nanoplate-like morphology of the samples.23
In Table S2 of the ESI,† BB1I2-4-110-12 and BB2I1-4-110-12 also have the larger BET value and pore volume. Thus, the large BET value and pore volume of the BiOpBrq/BiOmIn composites may play a role in enhancing the photocatalytic activity. The detailed BET and pore structure are described in the ESI.†
3.6 Photocatalytic activity
The changes in the UV-vis spectra during the photodegradation process of CV and phenol in the aqueous BiOpBrq/BiOmIn dispersions under visible light irradiation are illustrated in Fig. S12 of the ESI.† The degradation efficiency as a function of reaction time is illustrated in Fig. S13 of the ESI.† The removal efficiency is enhanced significantly in the presence of BiOpBrq/BiOmIn catalysts. After 48 h of irradiation, BiOpBrq/BiOmIn shows superior photocatalytic performance, with the CV removal efficiency up to 99%. To further understand the reaction kinetics of CV degradation, the apparent pseudo-first-order model24 expressed by the ln(Co/C) = kappt equation was applied in these experiments. Via the first-order linear fit from the data of Fig. S13† shown in Table 3, kapp of BB1I2-4-110-12 was obtained at the maximal degradation rate of 5.285 × 10−1 h−1, greatly higher than the other composites. Therefore, the Bi4O5Br2/BiOI composite shows the best photocatalytic activity. The result shows that the Bi4O5Br2/BiOI composite is a much more effective photocatalyst than the others. The superior photocatalytic ability of BiOpBrq/BiOmIn may be ascribed to its efficient utilization of visible light and the high separation efficiency of the electron–hole pairs with its composites.
Table 3 The pseudo-first-order rate constants for the degradation of CV with the BiOpBrq/BiOmIn photocatalysts under visible light irradiation
| pH |
Temp (°C) |
| 110 |
160 |
210 |
260 |
| k (h−1) |
R2 |
k (h−1) |
R2 |
k (h−1) |
R2 |
k (h−1) |
R2 |
| BB1I2 series |
| 1 |
0.171 |
0.96 |
0.137 |
0.95 |
0.185 |
0.96 |
0.100 |
0.97 |
| 4 |
0.374 |
0.95 |
0.323 |
0.96 |
0.528 |
0.99 |
0.239 |
0.99 |
| 7 |
0.064 |
0.97 |
0.126 |
0.99 |
0.080 |
0.96 |
0.106 |
0.96 |
| 10 |
0.129 |
0.97 |
0.201 |
0.95 |
0.077 |
0.95 |
0.189 |
0.96 |
| 13 |
0.013 |
0.96 |
0.029 |
0.97 |
0.042 |
0.96 |
0.022 |
0.96 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| BB2I1 series |
| 1 |
0.143 |
0.97 |
0.139 |
0.96 |
0.249 |
0.96 |
0.025 |
0.98 |
| 4 |
0.471 |
0.96 |
0.427 |
0.96 |
0.416 |
0.97 |
0.310 |
0.98 |
| 7 |
0.243 |
0.97 |
0.398 |
0.96 |
0.103 |
0.97 |
0.086 |
0.98 |
| 10 |
0.143 |
0.96 |
0.094 |
0.96 |
0.087 |
0.98 |
0.064 |
0.93 |
| 13 |
0.009 |
0.97 |
0.002 |
0.98 |
0.010 |
0.98 |
0.020 |
0.94 |
| BiOBr |
BiOI |
TiO2 |
| k (h−1) |
R2 |
k (h−1) |
R2 |
k (h−1) |
R2 |
| 0.037 |
0.97 |
0.093 |
0.99 |
0.057 |
0.99 |
The durability of the Bi4O5Br2/BiOI (BB1I2-4-210-12) composite was evaluated through recycling the used catalyst. There was no apparent loss of photocatalytic activity for removing crystal violet in the fifth cycle, and even in the tenth run, the declination in photocatalytic activity was less than 3% (Fig. 8(a)). The used Bi4O5Br2/BiOI was also examined by XRD, and there was no detectable difference between the as-prepared and used samples (Fig. 8(b)). Therefore, it can be deduced that the Bi4O5Br2/BiOI composite has good photostability.
 |
| | Fig. 8 (a) Cycling runs and (b) XRD patterns acquired before and after the photocatalytic degradation of CV in the presence of BB1I2-4-110-12. | |
3.7 Cathodoluminescence spectrum
To investigate the separation capacity of the photogenerated carriers in the heterostructures, the CL spectra of BiOBr, BiOI, and BiOpBrq/BiOmIn were measured and the results are given in Fig. 9 The characteristic emission peak around 2.01 eV nearly disappears for the BiOpBrq/BiOmIn heterostructure, indicating that the recombination of photogenerated charge carriers is inhibited greatly. The efficient charge separation could increase the lifetime of the charge carriers and enhance the efficiency of the interfacial charge transfer to the adsorbed substrates, and then improve the photocatalytic activity.
 |
| | Fig. 9 Photoluminescence spectra of TiO2, BiOBr, BiOI, and BiOpBrq/BiOmIn. Molar ratio KBr/KI (a) 1/2, (b) 2/1. | |
3.8 Separation and identification
With visible irradiation, temporal variations occurring in the solution of the CV dye during the degradation process were examined by HPLC-PDA-MS. Given the CV irradiation up to 24 h at pH 4, the chromatograms are illustrated in Fig. S14 of the ESI† and were recorded at 580, 350, and 300 nm, and nineteen intermediates were identified, with the retention time of under 50 min. The CV dye and its related intermediates are denoted as species A–J, a–f, and α–γ. Except for the initial CV dye (peak A), the peaks initially increase before subsequently decreasing, indicating the formation and transformation of intermediates.
In Fig. S15 of the ESI,† the maximum absorption of the spectral bands shifts from 588.5 nm (spectrum A) to 541.5 nm (spectrum J), from 377.0 nm (spectrum a) to 339.0 nm (spectrum f), and from 309.1 nm (spectrum α) to 278.3 nm (spectrum γ). The maximum adsorption in the visible and ultraviolet spectral region of each intermediate is depicted in Table S3.† They are identified as A–J a–f, and α–γ, respectively corresponding to the peaks A–J, a–f, and α–γ in Fig. S14 (ESI†). These shifts of the absorption band are presumed to result from the formation of a series of N-de-methylated intermediates. From these results, several families of intermediates could be distinguished. The intermediates were further identified using the HPLC-ESI mass spectrometric method, and the relevant mass spectra are illustrated in Fig. S16 and Table S3 (ESI†). The molecular ion peaks appear in the acid forms of the intermediates. The detailed data of the intermediates are described in the ESI.†
3.9 Mechanism of the photocatalytic degradation of CV
Generally speaking, three possible reaction mechanisms are suspected to be involved in dye photodegradation by a semiconductor, namely (i) a photolysis process, (ii) a dye photosensitization process, and (iii) a photocatalytic process.25 In this experiment, CV degradation by the photolysis process upon visible light irradiation in the blank experiment was not observable.
In this study, slight changes in the CV concentration over different samples can be detected during 30 min of a dark adsorption experiment before the photocatalytic reactions. The slight CV adsorption on the catalyst benefits the transfer of charge carriers between the dye and the catalyst surfaces in the dye photosensitization process. Presuming that the photosensitization process takes place with BiOpBrq/BiOmIn, that is to say, the photosensitization mechanism in the CV decomposition can not be neglected.
As is known, various primary reactive species, such as the hydroxyl radical HO˙, photogenerated hole h+, superoxide radical O2−˙ and singlet oxygen 1O2, can be formed during the photocatalytic degradation process in the UV-vis/semiconductor system.6,7,26,27 Shenawi-Khalil and his coworkers showed that the rhodamine-B and acetophenone photodegradation by BiOCl-bismuth oxyhydrate under visible light was dominated by O2−˙ and h+ oxidation as the main active species.25 Chen et al. proposed a pathway for generating active oxygen radicals (˙OH) on the surface of Bi2O2CO3/BiOI for the degradation of dye.28 Xiao’s group revealed that highly efficient visible-light-driven bisphenol-A removal with BiOBr/BiOI could be attributed to effective separation and transfer of the photoinduced charge carriers in BiOBr/BiOI with a narrower band gap and more negative conduction band position, which favored the photogenerated holes.23 Wang et al. reported that ˙OH radicals were generated by the multistep reduction of O2−˙.29 Therefore, photogenerated holes on the surface of bismuth oxyhalides were not expected to react with OH−/H2O to form ˙OH, suggesting that the decomposition of bisphenol-A18 and rhodamine27 could be attributed to a direct reaction with the photogenerated holes or with the superoxide radical or both species.
In order to evaluate the effect of the active species during the photocatalytic reaction, a series of quenchers were introduced to scavenge the relevant active species. O2˙−, ˙OH, h+, and 1O2 were investigated by adding 1.0 mM benzoquinone,30 isopropanol,31 ammonium oxalate,32 and sodium azide,33 respectively. As shown in Fig. 10(a), the degradation efficiency of IPA quenching decreased more than that of AO, and the degradation efficiency of BQ quenching decreased more than that of IPA, but the photocatalytic degradation of CV was not affected by the addition of SA. In short, the quenching effects of various scavengers showed that the reactive O2˙− played a major role, and ˙OH or h+ played a minor role in the CV degradation. From Fig. 10(b), the six characteristic peaks of the DMPO–O2− adducts were observed in the visible light irradiated BiOpBrq/BiOmIn dispersions. But, the four characteristic peaks of DMPO–OH adducts (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:2
2:2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartet pattern) were not observed in visible light irradiated BiOpBrq/BiOmIn aqueous dispersions. Fig. 10(b) indicated that no EPR signals were observed when the reaction was performed in the dark, while the signals with intensities corresponding to the characteristic peaks of DMPO–O2− adducts34 were observed during the reaction process under visible light irradiation, and the intensity gradually increased with the prolonged reaction time, suggesting that O2˙− (the major active species) has been formed in the presence of BiOpBrq/BiOmIn and oxygen under visible light irradiation.
1 quartet pattern) were not observed in visible light irradiated BiOpBrq/BiOmIn aqueous dispersions. Fig. 10(b) indicated that no EPR signals were observed when the reaction was performed in the dark, while the signals with intensities corresponding to the characteristic peaks of DMPO–O2− adducts34 were observed during the reaction process under visible light irradiation, and the intensity gradually increased with the prolonged reaction time, suggesting that O2˙− (the major active species) has been formed in the presence of BiOpBrq/BiOmIn and oxygen under visible light irradiation.
 |
| | Fig. 10 (a) The dye concentration during photodegradation as a function of irradiation time observed in BiOpBrq/BiOmIn under the addition of different scavengers: SA, IPA, AQ, and BQ. (b) DMPO spin-trapping EPR spectra for DMPO–O2− and DMPO–OH under visible light irradiation with BiOxCly/BiOmIn. | |
Wang et al. reported that ˙OH radicals were generated by the multistep reduction of O2−˙.29 However, photogenerated holes on the surface of bismuth oxyhalides were not expected to react with OH−/H2O to form ˙OH, suggesting that the decomposition of bisphenol-A18 and rhodamine27 could be attributed to a direct reaction with the photogenerated holes or with superoxide radical or both species. Therefore, the O2−˙ radical is the most important active species in the photocatalytic degradation of CV or phenol by BiOxCly/BiOmIn.
Chen et al. reported35 that Pt–TiO2 accumulated less negative species on catalyst surfaces, which deteriorated reaction rates, than pure TiO2 did in an acidic environment. The ˙OH radical is produced subsequently, as also shown in eqn (19)–(24).
| | |
O2−˙ + H+ + e− → HOO˙
| (19) |
| | |
HOO˙ + H2O → ˙OH + H2O2
| (20) |
| | |
H2O2 + e− → ˙OH + OH−
| (22) |
These cycles continuously occur when the system is exposed to visible light irradiation. Finally, after several cycles of photo-oxidation, the degradation of CV by the formed oxidant species can be expressed by eqn (25)–(27).
| | |
CV + h+ → CV+˙ → degraded compounds
| (25) |
| | |
CV + OH˙ / O2−˙ → degraded compounds
| (26) |
| | |
CV+˙ + OH˙/ O2−˙ → degraded compounds
| (27) |
It has been reported that some dyes exhibit a dye sensitized degradation mechanism.36 This photocatalytic degradation is also attributed to the photodegradation of CV through the photocatalytic pathway of CV photosensitized BiOpBrq/BiOmIn. CV absorbs a visible photon and is promoted to an excited electronic state CV*, from which an electron can be transferred into the conduction band of BiOpBrq/BiOmIn:
| | |
CV* + BiOpBrq/BiOmIn → CV+˙ + BiOpBrq/BiOmIn (e−)
| (29) |
Once the electron reaches the BiOpBrq/BiOmIn conduction band, it subsequently induces the generation of active oxygen species (eqn (30), (19)–(22)), which result in the degradation of CV. Clearly, apart from the photodegradation of CV through the pathway of BiOpBrq/BiOmIn-mediated and photosensitized processes, there is another kind of photocatalytic pathway to account for the enhanced photocatalytic activity. Both the photocatalytic process and the photosensitized process would work concurrently, as shown in Fig. 11.
 |
| | Fig. 11 The band structure diagram of the BiOpBrq/BiOmIn nanocomposites and the possible charge separation processes. | |
In earlier reports,5,35,37–39 the N-de-alkylation processes were preceded by the formation of a nitrogen-centered radical while the destruction of dye chromophore structures was preceded by the generation of a carbon-centered radical in the photocatalytic degradation of triphenylmethane dyes. On the basis of the above experimental results, a dye degradation mechanism is tentatively proposed, as depicted in Fig. S17–S19 (ESI†).
4. Conclusions
In the current process, the controllable crystal phases and morphologies of bismuth oxybromides/bismuth oxyiodides could be accomplished by simply changing some growth parameters, including the molar ratio (Br/I), pH value, and reaction temperature. The increased photocatalytic activities of BiOpBrq/BiOmIn could be attributed to the formation of the heterojunction between BiOpBrq and BiOmIn, which effectively suppresses the recombination of photoinduced electron–hole pairs. Both the photocatalytic process and the photosensitized process work concurrently. O2−˙ is the main active species and h+ and ˙OH are two minor active species in the whole process while 1O2 can be negligible. The reaction mechanisms for vis/BiOpBrq/BiOmIn proposed in this study should offer some insight for the future development of technology applications for the degradation of dyes.
Acknowledgements
This research was supported by the Ministry of Science and Technology of the Republic of China (NSC-101-2113 M- 142-001-MY3).
Notes and references
- C. J. Brinker, B. Cornils and M. Bonet, Triarylmethane and Diarylmethane Dyes, Wiley-VCH, New York, 6th edn, 2001 Search PubMed
 .
. - L. M. Lewis and G. L. Indig, J. Photochem. Photobiol., B, 2002, 67, 139–148 CrossRef CAS
 .
. - B. P. Cho, T. Yang, L. R. Blankenship, J. D. Moody, M. Churchwell, F. A. Bebland and S. J. Culp, Chem. Res. Toxicol., 2003, 16, 285–294 CrossRef CAS PubMed
 .
. - K. Yu, S. G. Yang, C. Liu, H. Chen, H. Li, C. Sun and A. B. Stephen, Environ. Sci. Technol., 2012, 46, 7318–7326 CrossRef CAS PubMed
 .
. - Y. H. Liao, J. X. Wang, J. S. Lin, W. H. Chung, W. Y. Lin and C. C. Chen, Catal. Today, 2011, 174, 148–159 CrossRef CAS PubMed
 .
. - Y. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, Chem. Sci., 2012, 3, 2812–2822 RSC
 .
. - M. Q. Yang, Y. Zhang, N. Zhang, Z. R. Tang and Y. J. Xu, Sci. Rep., 2013, 3, 3314 Search PubMed
 .
. - M. Q. Yang, N. Zhang, M. Pagliaro and Y. J. Xu, Chem. Soc. Rev., 2014, 43, 8240–8254 RSC
 .
. - N. Zhang, Y. Zhang and Y.-J. Xu, Nanoscale, 2012, 4, 5792–5813 RSC
 .
. - N. Zhang, M. Q. Yang, Z. R. Tang and Y. J. Xu, ACS Nano, 2014, 8, 623–633 CrossRef CAS PubMed
 .
. - Y. Zhang, Z. R. Tang, X. Fu and Y. J. Xu, ACS Nano, 2010, 4, 7303–7311 CrossRef CAS PubMed
 .
. - C. Han, M. Q. Yang, B. Weng and Y. J. Xu, Phys. Chem. Chem. Phys., 2014, 16, 16891–16903 RSC
 .
. - W. W. Lee, C. S. Lu, C. W. Chuang, Y. J. Chen, J. Y. Fu, C. W. Siao and C. C. Chen, RSC Adv., 2015, 5, 23450–23463 RSC
 .
. - L. Ye, J. Chen, L. Tian, J. Liu, T. Peng, K. Deng and L. Zan, Appl. Catal., B, 2013, 130–131, 1–7 CAS
 .
. - Y. R. Jiang, H. P. Lin, W. H. Chung, Y. M. Dai, W. Y. Lin and C. C. Chen, J. Hazard. Mater., 2015, 283, 787–805 CrossRef CAS PubMed
 .
. - S. T. Huang, Y. R. Jiang, S. Y. Chou, Y. M. Dai and C. C. Chen, J. Mol. Catal. A: Chem., 2014, 391, 105–120 CrossRef CAS PubMed
 .
. - S. Shenawi-Khalil, V. Uvarov, S. Fronton, I. Popov and Y. Sasson, Appl. Catal., B, 2012, 117–118, 148–155 CrossRef CAS PubMed
 .
. - X. Xiao, R. Hao, M. Liang, X. Zuo, J. Nan, L. Li and W. Zhang, J. Hazard. Mater., 2012, 233–234, 122–130 CrossRef CAS PubMed
 .
. - J. Cao, B. Xu, B. Luo, H. Lin and S. Chen, Catal. Commun., 2011, 13, 63–68 CrossRef CAS PubMed
 .
. - X. Xiao, R. Hao, X. Zuo, J. Nan, L. Li and W. Zhang, Chem. Eng. J., 2012, 209, 293–300 CrossRef CAS PubMed
 .
. - X. Xiao and W. D. Zhang, RSC Adv., 2011, 1, 1099–1105 RSC
 .
. - J. Zhang, F. Shi, J. Lin, D. Chen, J. Gao, Z. Huang, X. Ding and C. Tang, Chem. Mater., 2008, 20, 2937–2941 CrossRef CAS
 .
. - J. Wang, Y. Yu and L. Zhang, Appl. Catal., B, 2013, 136–137, 112–121 CrossRef CAS PubMed
 .
. - W. D. Wang, F. Q. Huang and X. P. Lin, Scr. Mater., 2007, 56, 669–672 CrossRef CAS PubMed
 .
. - S. K. Sanaa, U. Vladimir, M. Ella, P. Inna and S. Yoel, Appl. Catal., A, 2012, 413–414, 1–9 Search PubMed
 .
. - X. Xiao, R. Hu, C. Liu, C. Xing, X. Zuo, J. Nan and L. Wang, Chem. Eng. J., 2013, 225, 790–797 CrossRef CAS PubMed
 .
. - S. K. Sana, U. Vladimir, F. Sveta, P. Inna and S. Yoel, Appl. Catal., B, 2012, 117–118, 148–155 Search PubMed
 .
. - L. Chen, S. F. Yin, S. L. Luo, R. Huang, Q. Zhang, T. Hong and P. C. T. Au, Ind. Eng. Chem. Res., 2012, 51, 6760–6768 CrossRef CAS
 .
. - J. Wang, Y. Yu and L. Zhang, Appl. Catal., B, 2013, 136–137, 112–121 CrossRef CAS PubMed
 .
. - M. C. Yin, Z. S. Li, J. H. Kou and Z. G. Zou, Environ. Sci. Technol., 2009, 43, 8361–8366 CrossRef CAS PubMed
 .
. - L. S. Zhang, K. H. Wong, H. Y. Yip, C. Hu, J. C. Yu, C. Y. Chan and P. K. Wong, Environ. Sci. Technol., 2010, 44, 1392–1398 CrossRef CAS PubMed
 .
. - S. G. Meng, D. Z. Li, M. Sun, W. J. Li, J. X. Wang, J. Chen, X. Z. Fu and G. C. Xiao, Catal. Commun., 2011, 12, 972–975 CrossRef CAS PubMed
 .
. - G. Li, K. H. Wong, X. Zhang, C. Hu, J. C. Yu, R. C. Y. Chan and P. K. Wong, Chemosphere, 2009, 76, 1185–1191 CrossRef CAS PubMed
 .
. - X. Xiao, C. Xing, G. He, X. Zuo, J. Nan and L. Wang, Appl. Catal., B, 2014, 148–149, 154–163 CrossRef CAS PubMed
 .
. - H. J. Fan, C. S. Lu, W. L. W. Lee, M. R. Chiou and C. C. Chen, J. Hazard. Mater., 2011, 185, 227–235 CrossRef CAS PubMed
 .
. - X. Zhu, J. Zhang and F. Chen, Appl. Catal., B, 2011, 102, 316–322 CrossRef CAS PubMed
 .
. - H. P. Lin, J. Y. Chen, W. H. Lien and C. C. Chen, J. Taiwan Inst. Chem. Eng., 2014, 45, 2469–2479 CrossRef PubMed
 .
. - K. L. Li, W. W. Lee, C. S. Lu, Y. M. Dai, S. Y. Chou, M. C. Wang and C. C. Chen, J. Taiwan Inst. Chem. Eng., 2014, 45, 2688–2697 CrossRef CAS PubMed
 .
. - H. L. Chen, W. W. Lee, W. H. Chung, Y. J. Chen, Y. R. Jiang, H. P. Lin, W. Y. Lin and C. C. Chen, J. Taiwan Inst. Chem. Eng., 2014, 45, 1892–1909 CrossRef CAS PubMed
 .
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra01702e |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), pH value, temperature, and time, different BiOpBrq/BiOmIn composites could be synthesized and were labeled as in Table S1 of the ESI,† namely BB1I2-1-110-12 (Br
2), pH value, temperature, and time, different BiOpBrq/BiOmIn composites could be synthesized and were labeled as in Table S1 of the ESI,† namely BB1I2-1-110-12 (Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = 1
I = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; pH = 1; temp = 110 °C; time = 12 h) to BB2I1-13-260-12 (Br
2; pH = 1; temp = 110 °C; time = 12 h) to BB2I1-13-260-12 (Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = 2
I = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; pH = 13; temp = 260 °C; time = 12 h) for the as-prepared samples, respectively.
1; pH = 13; temp = 260 °C; time = 12 h) for the as-prepared samples, respectively.

 BiOBr;
BiOBr;  Bi4O5Br2;
Bi4O5Br2;  Bi24O31Br10;
Bi24O31Br10;  Bi3O4Br;
Bi3O4Br;  Bi12O17Br2;
Bi12O17Br2;  Bi5O7Br;
Bi5O7Br;  BiOI;
BiOI;  Bi7O9I3;
Bi7O9I3;  Bi5O7I)
Bi5O7I)






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:2
2:2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 quartet pattern) were not observed in visible light irradiated BiOpBrq/BiOmIn aqueous dispersions. Fig. 10(b) indicated that no EPR signals were observed when the reaction was performed in the dark, while the signals with intensities corresponding to the characteristic peaks of DMPO–O2− adducts34 were observed during the reaction process under visible light irradiation, and the intensity gradually increased with the prolonged reaction time, suggesting that O2˙− (the major active species) has been formed in the presence of BiOpBrq/BiOmIn and oxygen under visible light irradiation.
1 quartet pattern) were not observed in visible light irradiated BiOpBrq/BiOmIn aqueous dispersions. Fig. 10(b) indicated that no EPR signals were observed when the reaction was performed in the dark, while the signals with intensities corresponding to the characteristic peaks of DMPO–O2− adducts34 were observed during the reaction process under visible light irradiation, and the intensity gradually increased with the prolonged reaction time, suggesting that O2˙− (the major active species) has been formed in the presence of BiOpBrq/BiOmIn and oxygen under visible light irradiation.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.









































