Light emitting diodes (LEDs) encapsulation of polymer composites based on poly(propylene fumarate) crosslinked with poly(propylene fumarate)-diacrylate
Abstract
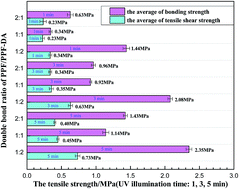
A novel poly(propylene fumarate)-based polymer networks with good performance for high-power light emitting diodes (LEDs) encapsulation was introduced in this research. Polymer networks have been prepared by radical polymerization using poly(propylene fumarate) (PPF) and poly(propylene fumarate)-diacrylate (PPF-DA) macromers with photo-initiator systems. Photo-crosslinking was accomplished with BAPO accelerated by UV irradiation. It provided an effective curing behavior. PPF and PPF-DA were characterized by Fourier-transform infrared spectroscopy and 1H NMR. The thermal gravity analysis showed that the PPF/PPF-DA (double bond ratios 0.5, 1, and 2) encapsulation material were stable below 287.98 °C, 285.26 °C and 271.60 °C, respectively. The mechanical properties experiments indicated that bonding strength was in the range of 1.09 ± 0.04 MPa to 2.39 ± 0.04 MPa and tensile-shear strength ranged from 0.38 ± 0.02 MPa to 0.79 ± 0.03 MPa. The cured PPF/PPF-DA networks can be used as a LEDs encapsulant, owing to suitable refractive index (n = 1.537–1.541), high transparency (98.75%), appropriate tensile strength, and excellent thermal stability.

 Please wait while we load your content...
Please wait while we load your content...